Coming of Age in the Milky Way (42 page)
Read Coming of Age in the Milky Way Online
Authors: Timothy Ferris
Tags: #Science, #Philosophy, #Space and time, #Cosmology, #Science - History, #Astronomy, #Metaphysics, #History
Lesser stars contribute less dramatically to the chemical evolution of the universe, but they too play their part, wafting the nuclei of heavy elements into space through stellar winds, shedding their outer atmospheres as planetary nebulae, or blowing them into space in the less disruptive but still imposing explosions called novae. One can see their handiwork in the chemical gradients that show up across the faces of galaxies: Metals are scarce in the spectra of stars near the galactic center, where few stars have formed since the early days, while stars in the spiral arms, where star formation continues apace, are rich in these heavier elements. We see and touch—indeed,
are
—the products of the evolution of atoms and stars.
Much remains to be learned about dying stars and their chemical legacies. We conclude this unfinished story with a coda by the Berkeley astronomer Frank Shu, drawing on research by the Soviet scientists Yakob Zel’dovich and Igor Novikov. Writes Shu:
Stars begin their lives as a mixture mostly of hydrogen nuclei and their stripped electrons. During a massive star’s luminous phase, the protons are combined by a variety of complicated reactions into heavier and heavier elements. The nuclear binding energy released this way ultimately provides entertainment and employment for astronomers. In the end, however, the supernova process serves to undo most of this nuclear evolution. In the end, the core forms a mass of neutrons. Now, the final state, neutrons, contains
less
nuclear binding energy than the initial state, protons, and electrons. So where did all the energy come from when the star was shining all those millions of years? Where did the energy come from to produce the sound and the fury which is a supernova explosion? Energy is conserved: who paid the debts at the end? Answer: Gravity! The gravitational potential of the final neutron star is much greater (negatively: that’s the debt) than the gravitational potential energy of the corresponding main-sequence star. So, despite all the intervening interesting nuclear physics, ultimately Kelvin and Helmholtz were right after all! The ultimate energy source in the stars which produce the greatest amount of energy is gravity power.
16
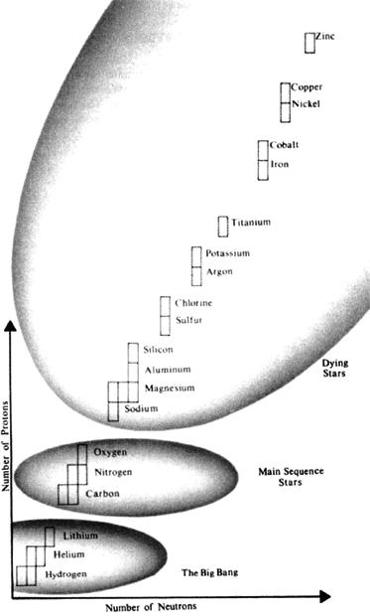
Cosmic evolution of elements involves the building of simple atomic nuclei in the big bang, and the subsequent fusion of these light nuclei into heavier and more complex nuclei inside stars. (After Reeves, 1984.)
Let that be the human image we call to mind as we watch the gold dust and diamonds bid farewell to the exhausted star as they parade away to be woven into future worlds and minds: The face of Kelvin, the old boy who cowed Rutherford one day when the century was young, shaking off his sleep to scowl, then smile.
*
After many false starts, Cannon designated the classes by the letters O, B, A, F, G, K, and M. Students ever since, in a largely unconscious tribute to her memory, have learned the sequence via the mnemonic phrase “Oh, Be a Fine Girl, Kiss Me.”
*
There is a quantum chance that a real cannonball will do the same thing, but as this requires nearly all its protons to get lucky at once, the odds of its happening are small. One can calculate, via quantum theory, that it has almost certainly never occurred, anywhere in the universe, even if—unhappy thought—cannonballs and fortress walls are a cosmic commonplace.
*
Carl Friedrich von Weizsäcker, the physicist and later philosopher of science, had shown how the proton-proton reaction would work, but had not calculated its energy output for a solar-mass star.
*
To explain why the galaxies are not infinitely far apart in an infinitely old, expanding universe, the theory proposed that hydrogen atoms materialize spontaneously, out of empty space, and thence condense into new stars and galaxies. This hypothesis, though much ridiculed at the time, is not so implausible as it might at first appear. Owing to quantum indeterminacy, “virtual” particles do materialize out of space all the time, though their lifetimes normally are short. In some versions of the big bang theory, notably the “inflationary universe” model,
all
matter is said to have appeared out of a vacuum—though all at once, and long ago.
REATION
O landless void, O skyless void,
O nebulous, purposeless space,
Eternal and timeless,
Become the world, extend!—Tahitian creation tale
What really interests me is whether God had any choice in the creation of the world.
—Einstein
T
HE
Q
UANTUM AND
I
TS
D
ISCONTENTS
What is the path? There is no path.
—Niels Bohr, quoting Goethe
Progress in physics has always moved from the intuitive toward the abstract.
—Max Born
T
he act of exploration alters the perspective of the explorer; Odysseus and Marco Polo and Columbus returned home as changed men. So it has been with scientific investigation of the extremities of scale, from the grand sweep of cosmological space down to the cramped and frantic world of the subatomic particles: These journeys changed us, challenging many of the scientific and philosophical conceptions we had most cherished. Some had to be discarded, like baggage left behind on a trek across a desert. Others were altered and repaired almost beyond recognition, like the veteran mountaineer’s hand-hammered pitons or the old seaman’s knife with its twine-encrusted handle and bone-thin blade. Exploration of the realm of the galaxies extended the reach of human vision by a factor of some 10
26
larger than the human scale, and brought about the revolution we identify with relativity, which revealed that the
Newtonian world view was but a parochialism in a wider universe where space is curved and time becomes pliant. Exploration of the subatomic realm carried us far into the realm of the small, to some 10
•15
of the human scale, and it, too, wrought a revolution. This was
quantum
physics, and all that it touched it transformed.
Quantum theory was born in 1900, when Max Planck realized that he could account for what was called the black-body curve—the spectrum of energy generated by a perfectly radiating object —only if he abandoned the classical assumption that energy is emitted continuously and replaced it with the unprecedented hypothesis that energy comes in discrete units. Planck called these units
quanta
, after the Greco-Latin word for “how much” (as in
quantity)
, and he defined them in terms of the quantum of action, symbolized by the letter
h
. Planck was no revolutionary—at age forty-two he was an old man by the standards of mathematical science, and a pillar of nineteenth-century German high culture to boot—but he readily appreciated that the quantum principle would shatter much of the classical physics to which he had devoted his career. “The greater [its] difficulties,” he wrote, “… the more significant, it finally will show itself to be for the broadening and deepening of our whole knowledge in physics.”
1
His words proved prophetic: Constantly changing and developing, altering its coloration as unpredictably as a reflection in a soap bubble, quantum physics soon expanded into virtually every area of physics, and Planck’s
h
came to be regarded as a fundamental constant of nature, on a par with Einstein’s
c
, the velocity of light.
The quantum principle was very strange—it was, as Gamow remarked, as if one could drink a pint of beer or no beer at all, but were barred by a law of nature from drinking any quantity of beer between zero and one pint—and it got stranger as it evolved. The decisive break with classical physics came in 1927, when the young German physicist Werner Heisenberg arrived at the indeterminacy principle. Heisenberg found that one can learn either the exact position of a given particle or its exact trajectory,
but not both
. If, for instance, we watch a proton fly through a cloud chamber, we can by recording its track discern the direction in which it is moving, but in the process of plowing through the water vapor in the chamber the proton will have slowed down, robbing us of information about just where it was at any given instant. Alternately, we can irradiate the proton—take a flash photograph of it, so to speak—and
thus determine its exact location at a given instant, but the light or other radiation we employ to take the photograph will knock the proton off its appointed rounds, depriving us of precise knowledge of where it would have gone had we left it alone. We are, therefore, limited in our knowledge of the subatomic world: We can extract only partial answers, the nature of which are decided to some extent by the questions we choose to ask. When Heisenberg calculated the inescapable minimum amount of uncertainty that limits our understanding of events on the small scale, he found that it is defined by nothing other than
h
, Planck’s quantum of action.

The Scale of the Known Universe
| Radius (meters) | Characteristic Objects |
| 10 26 | Observable universe |
| 10 24 | Superclusters of galaxies |
| 10 23 | Clusters of galaxies |
| 10 22 | Groups of galaxies (e.g., the Local Group) |
| 10 21 | Milky Way galaxy |
| 10 18 | Giant nebulae, molecular clouds |
| 10 12 | Solar system |
| 10 11 | Outer atmospheres of red giant stars |
| 10 9 | Sun |
| 10 8 | Giant planets (e.g., Jupiter) |
| 10 7 | Dwarf stars, Earthlike planets |
| 10 5 | Asteroids, comet nuclei |
| 10 4 | Neutron stars |
| 1 | Human beings |
| 10 −2 | DNA molecules (long axis) |
| 10 −5 | Living cells |
| 10 −9 | DNA molecules (short axis) |
| 10 −10 | Atoms |
| 10 −14 | Nuclei of heavy atoms |
| 10 −15 | Protons, neutrons |
| 10 −35 | Planck length: Quantum of space; radius of “dimensionless” particles in string theory |
