Read In Pursuit of the Unknown Online
Authors: Ian Stewart
In Pursuit of the Unknown (39 page)
Fig 46
Hero's aeolipile.
Watt learned that steam could be a source of power in 1762 when he was 26 years old. He didn't discover it watching a kettle: his friend John Robison, a professor of natural philosophy at the University of Edinburgh, told him about it. But practical steam power was much older. Its discovery is often credited to the Italian engineer and architect Giovanni Branca, whose
Le Machine
(âMachine') of 1629 contained 63 woodcuts of mechanical gadgets. One shows a paddlewheel that would spin on its axle when steam from a pipe collided with its vanes. Branca speculated that this machine might be useful for grinding flour, lifting water, and cutting up wood, but it was probably never built. It was more of a thought experiment, a mechanical pipedream like Leonardo da Vinci's flying machine.
In any case, Branca was anticipated by Taqi al-Din Muhammad ibn Ma'ruf al-Shami al-Asadi, who lived around 1550 in the Ottoman Empire and was widely held to be the greatest scientist of his age. His achievements are impressive. He worked in everything from astrology to zoology, including clock-making, medicine, philosophy, and theology, and he wrote over 90 books. In his 1551
Al-turuq al-samiyya fi al-alat al-ruhaniyya
(âThe Sublime Methods of Spiritual Machines'), al-Din described a primitive steam turbine, saying that it could be used to turn roasting meat on a spit.
The first truly practical steam engine was a water pump invented by Thomas Savery in 1698. The first to make commercial profits, built by Thomas Newcomen in 1712, triggered the Industrial Revolution. But Newcomen's engine was very inefficient. Watt's contribution was to introduce a separate condenser for the steam, reducing heat loss. Developed using money provided by the entrepreneur Matthew Bolton, this new type of engine used only a quarter as much coal, leading to huge savings. Boulton and Watt's machine went into production in 1775, more than 220 years after al-Din's book. By 1776, three were up and running: one in a coal mine at Tipton, one in a Shropshire ironworks, and one in London.
Steam engines performed a variety of industrial tasks, but by far the commonest was pumping water from mines. It cost a lot of money to develop a mine, but as the upper layers became worked out and operators were forced to dig deeper into the ground, they hit the water table. It was worth spending quite a lot of money to pump the water out, since the
alternative was to close the mine and start again somewhere else â and that might not even be feasible. But no one wanted to pay more than they had to, so a manufacturer who could design and build a more efficient steam engine would corner the market. So the basic question of how efficient a steam engine could be cried out for attention. Its answer did more than just describe the limits to steam engines: it created a new branch of physics, whose applications were almost boundless. The new physics shed light on everything from gases to the structure of the entire universe; it applied not just to the dead matter of physics and chemistry, but perhaps also to the complex processes of life itself. It was called thermodynamics: the motion of heat. And, just as the law of conservation of energy in mechanics ruled out mechanical perpetual motion machines, the laws of thermodynamics ruled out similar machines using heat.
One of those laws, the first law of thermodynamics, revealed a new form of energy associated with heat, and extended the law of conservation of energy (
Chapter 3
) into the new realm of heat engines. Another, without any previous precedent, showed that some potential ways to exchange heat, which did not conflict with conservation of energy, were nevertheless impossible because they would have to create order from disorder. This was the second law of thermodynamics.
Thermodynamics is the mathematical physics of gases. It explains how large-scale features like temperature and pressure arise from the way the gas molecules interact. The subject began with a series of laws of nature relating temperature, pressure, and volume. This version is called classical thermodynamics, and did not involve molecules â at that time few scientists believed in them. Later, the gas laws were underpinned by a further layer of explanation, based on a simple mathematical model explicitly involving molecules. The gas molecules were thought of as tiny spheres that bounced off each other like perfectly elastic billiard balls, with no energy being lost in the collision. Although molecules are not spherical, this model proved to be remarkably effective. It is called the kinetic theory of gases, and it led to experimental proof that molecules exist.
The early gas laws emerged in fits and starts over a period of nearly fifty years, and are mainly attributed to the Irish physicist and chemist Robert Boyle, the French mathematician and balloon pioneer Jacques Alexandre César Charles, and the French physicist and chemist Joseph Louis Gay-Lussac. However, many of the discoveries were made by others. In 1834,
the French engineer and physicist Ãmile Clapeyron combined all of these laws into one, the ideal gas law, which we now write as
pV
=
RT
Here
p
is pressure,
V
is volume,
T
is the temperature, and
R
is a constant. The equation states that pressure times volume is proportional to temperature. It took a lot of work with many different gases to confirm each separate law, and Clapeyron's overall synthesis, experimentally. The word âideal' appears because real gases do not obey the law in all circumstances, especially at high pressures where interatomic forces come into play. But the ideal version was good enough for designing steam engines.
Thermodynamics is encapsulated in a number of more general laws, not reliant on the precise form of the gas law. However, it does require there to be some such law, because temperature, pressure, and volume are not independent. There has to be some relation between them, but it doesn't greatly matter what.
The first law of thermodynamics stems from the mechanical law of conservation of energy. In
Chapter 3
we saw that there are two distinct kinds of energy in classical mechanics: kinetic energy, determined by mass and speed, and potential energy, determined by the effect of forces such as gravity. Neither of these types of energy is conserved on its own. If you drop a ball, it speeds up, thereby gaining kinetic energy. It also falls, losing potential energy. Newton's second law of motion implies that these two changes cancel each other out exactly, so the total energy does not change during the motion.
However, this is not the full story. If you put a book on a table and give it a push, its potential energy doesn't change provided the table is horizontal. But its speed does change: after an initial increase produced by the force with which you pushed it, the book quickly slows down and comes to rest. So its kinetic energy starts at a nonzero initial value just after the push, and then drops to zero. The total energy therefore also decreases, so energy is not conserved. Where has it gone? Why did the book stop? According to Newton's first law, the book should continue to move, unless some force opposes it. That force is friction between the book and the table. But what is friction?
Friction occurs when rough surfaces rub together. The rough surface of the book has bits that stick out slightly. These come into contact with parts of the table that also stick out slightly. The book pushes against the table, and the table, obeying Newton's third law, resists. This creates a force that
opposes the motion of the book, so it slows down and loses energy. So where does the energy go? Perhaps conservation simply does not apply. Alternatively, the energy is still lurking somewhere, unnoticed. And that's what the first law of thermodynamics tells us: the missing energy appears as heat. Both book and table heat up slightly. Humans have known that friction creates heat even since some bright spark discovered how to rub two sticks together and start a fire. If you slide down a rope too fast, your hands get rope burns from the friction. There were plenty of clues. The first law of thermodynamics states that heat is a form of energy, and energy â thus extended â is conserved in thermodynamic processes.
The first law of thermodynamics places limits on what you can do with a heat engine. The amount of kinetic energy that you can get out, in the form of motion, cannot be more than the amount of energy you put in as heat. But it turned out that there is a further restriction on how efficiently a heat engine can convert heat energy into kinetic energy; not just the practical point that some of the energy always gets lost, but a theoretical limit that prevents all of the heat energy being converted to motion. Only some of it, the âfree' energy, can be so converted. The second law of thermodynamics turned this idea into a general principle, but it will take a while before we get to that. The limitation was discovered by Nicolas Léonard Sadi Carnot in 1824, in a simple model of how a steam engine works: the Carnot cycle.
To understand the Carnot cycle it is important to distinguish between heat and temperature. In everyday life, we say that something is hot if its temperature is high, and so confuse the two concepts. In classical thermodynamics, neither concept is straightforward. Temperature is a property of a fluid, but heat makes sense only as a measure of the transfer of energy between fluids, and is not an intrinsic property of the state (that is, the temperature, pressure, and volume) of the fluid. In the kinetic theory, the temperature of a fluid is the average kinetic energy of its molecules, and the amount of heat transferred between fluids is the change in the total kinetic energy of their molecules. In a sense heat is a bit like potential energy, which is defined relative to an arbitrary reference height; this introduces an arbitrary constant, so âthe' potential energy of a body is not uniquely defined. But when the body changes height, the difference in potential energies is the same whatever reference height is used, because the constant cancels out. In short, heat measures changes, but temperature measures states. The two are linked: heat transfer is possible only when the
fluids concerned have different temperatures, and then it is transferred from the hotter one to the cooler one. This is often called the Zeroth law of thermodynamics because logically it precedes the first law, but historically it was recognised later.
Temperature can be measured using a thermometer, which exploits the expansion of a fluid, such as mercury, caused by increased temperature. Heat can be measured by using its relation to temperature. In a standard test fluid, such as water, every 1-degree rise in temperature of 1 gram of fluid corresponds to a fixed increase in the heat content. This amount is called the specific heat of the fluid, which in water is 1 calorie per gram per degree Celsius. Note that heat
increase
is a change, not a state, as required by the definition of heat.
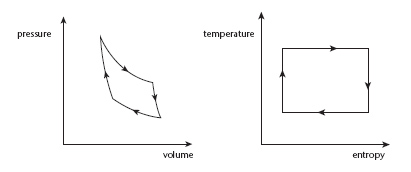
We can visualise the Carnot cycle by thinking of a chamber containing gas, with a movable piston at one end. The cycle has four steps:
1
Heat the gas so rapidly that its temperature doesn't change. It expands, performing work on the piston.
2
Allow the gas to expand further, reducing the pressure. The gas cools.
3
Compress the gas so rapidly that its temperature doesn't change. The piston now performs work on the gas.
4
Allow the gas to expand further, increasing the pressure. The gas returns to its original temperature.
In a Carnot cycle, the heat introduced in the first step transfers kinetic energy to the piston, allowing the piston to do work. The quantity of energy transferred can be calculated in terms of the amount of heat introduced and the temperature difference between the gas and its surroundings. Carnot's theorem proves that in principle a Carnot cycle is the most efficient way to convert heat into work. This places a stringent limit on the efficiency of any heat engine, and in particular on a steam engine.
In a diagram showing the pressure and volume of the gas, a Carnot cycle looks like
Figure 47
(
left
). The German physicist and mathematician Rudolf Clausius discovered a simpler way to visualise the cycle,
Figure 47
(
right
). Now the two axes are temperature and a new and fundamental quantity called
entropy
. In these coordinates, the cycle becomes a rectangle, and the amount of work performed is just the area of the rectangle.