Life on a Young Planet (15 page)
Read Life on a Young Planet Online
Authors: Andrew H. Knoll
Could life have begun as a film on fool’s gold? We don’t know, but recent laboratory experiments lend support to at least parts of Wächtershäuser’s hypothesis. Among other things, Wächtershäuser and his colleagues have generated acetic acid by the chemical fixation of carbon monoxide on a slurry of iron and nickel sulfides. These slurries also catalyze the formation of peptide chains from activated amino acids. Many aspects of the metabolism-first hypothesis remain to be tested, and the scenario still faces the formidable problem of integrating metabolism with nucleic acids and proteins. The experiments do, however, suggest that we should approach unconventional hypotheses about prebiotic evolution with an open mind. Mao Zedong may not have meant it when he urged fellow revolutionaries to “let a thousand flowers bloom,” but in research on the origin of life, we need all the ideas we can muster.
Once genes, proteins, and membranes were in place, life probably climbed the trunk of Darwin’s great Tree of Life quickly, propelled by natural selection, gene duplication, and the lateral transfer of genes. Biological expansion required that genes take control over many functions, but it isn’t necessary to believe that all genetic takeover occurred in one cell line. More likely, biochemical innovations arose individually in a number of distinct lineages. One line may have made vitamin B, another fatty acids, and a third proteins that catalyze replication. In the leaky world of protocells, the gene products of one cell may have been available to all, leading to complex communities linked by biosynthetic codependence.
(We still live in such a world—you need your morning orange juice because your cells cannot synthesize vitamin C.) Leaky membranes would have permitted genes as well as gene products to pass from one cell to another, allowing diverse biochemical pathways to aggregate in a small number of lineages destined to spread across the world.
Early metabolisms must have been simple, employing generalized enzymes that catalyzed many reactions with low efficiency. With time, however, natural selection produced enzymes that were both efficient and specific. For example, the first bacteria to use sulfate in respiration undoubtedly did so poorly, thriving largely because nobody else could do it at all. At first, sulfate would have been harnessed using enzymes that also served other functions, but as new variants better able to reduce sulfate outcompeted their compatriots, selection for efficient sulfate reduction progressed rapidly. Of course, this selection would have come at a cost, because the better that enzymes functioned in their new role, the more poorly they catalyzed other reactions.
Thus, as selection honed enzymatic function, evolving cells would have needed a source of new genes. Lateral transfer helped, although gene sharing probably slowed as increasingly competent membranes evolved. A second source, of continuing importance, was gene duplication. Errors in replication can result in extra copies of genes, providing raw material for evolutionary innovation. Once duplicated, the two gene copies can respond to differing selection pressures, eventually giving rise to two enzymes with distinct functions.
It is relatively easy to see how selection could have driven the evolution of specific enzymes, but what about complex metabolic pathways that integrate the activities of many proteins? The molecular intricacy of photosynthesis rivals the anatomical complexity of vertebrate eyes, chosen by Darwin to illustrate how “organs of extreme perfection” could evolve. Darwin’s creationist critics believed that the complexities of the eye must reflect intelligent design, but Darwin knew better. Among living organisms, he noted, we find a spectrum of photosensitive structures that runs from simple eyespots (pigment concentrations in single cells) to eyes with muscles, lenses, and optical nerves. All meet the functional demands of their bearers. Thus, Darwin argued that natural selection can fashion complexity from simplicity as long as all intermediates are functional.
The same is true of photosynthesis. At first glance, the photosynthetic apparatus may appear to be nature’s most elegant molecular assembly, but closer inspection reveals it to be her most wonderfully contrived Rube Goldberg machine, its impressive complexity resolvable into a series of components, each with its own origin and evolution.
To begin with, chlorophyll, the central pigment in photosynthesis, appears to have evolved from simpler but functional precursors. The earliest of these were probably present in the prebiotic environment, whereas later transitional forms still occur as chemical intermediates in chlorophyll biosynthesis. As the biosynthetic pathway of chlorophyll evolved, each new step modified the function of the previous end product.
Chlorophyll, in turn, is linked with proteins in an intricate molecular assembly called a photosystem that turns sunlight into chemical energy (
figure 5.4
). Once again, present-day complexity appears to have evolved by gene duplication and lateral transfer. Gene duplication elaborated the families of proteins that transport electrons in photosynthesis, whereas the paired photosystems of cyanobacteria (and green plants) originated in separate groups of bacteria and came together by lateral transfer. (All the genes required to build and operate a photosystem occur together along a strand of DNA. Thus packaged, this functional cassette of genes appears to have migrated from one photosynthetic bacterium to another, perhaps aided by viruses or by uptake from dead cells.)
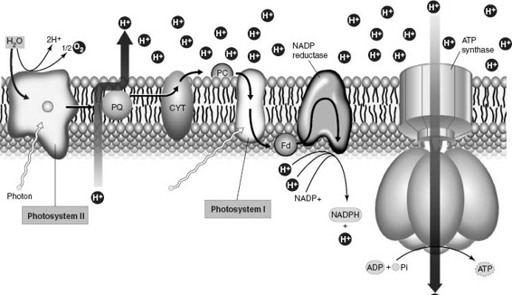
Figure 5.4.
Diagram showing the molecular assembly of photosystems in cyanobacteria and green plants. Chlorophyll and other pigments absorb photons of light and transfer their energy to “excited” electrons. The electrons are then passed in bucket-brigade fashion along a chain of proteins embedded in the photosynthetic membrane. This set of chemical reactions culminates in the formation of ATP and NADPH, molecules that supply the chemical power needed to fix carbon dioxide into sugar (in a separate set of reactions that takes place outside the photosynthetic membrane). Photosynthetic bacteria have one photosystem of linked pigments and proteins. As shown here, cyanobacteria and green plants use two complete photosystems that work together; the chemical breakdown of water in Photosystem II provides the electrons needed for photosynthesis. (Reproduced with permission from W. K. Purves, D. Sadawa, G. H. Orians, and H. C. Heller, 2001.
Life: The Science of Biology
, Sixth Edition, Sinauer Associates and W. H. Freeman and Company)
In metabolism, then, as in proteins, membranes, and nucleic acids, we can envision simple beginnings based on naturally occurring molecules, biological emancipation as biosynthetic pathways evolved, and, finally, complex biochemistry shaped by natural selection, gene duplication, and lateral transfer. The generative power of these processes is astonishing.
We are not close to solving the riddle of life’s origins. Origin-of-life research resembles a maze with many entries, and we simply haven’t traveled far enough down most routes to know which ones end in blind alleys. Yet, increasingly, chemists and molecular biologists have abandoned the early view that life originated by means of improbable reactions that came to pass only because vast intervals of time were available. Most now believe that life’s origin (or origins—it could have happened more than once) involved chemistry that was both probable and efficient; there is a direct route through the maze, if only we can find it.
While we have no sharp constraints on the timetable of prebiotic evolution, it appears that by 3.8 billion years ago life may already have gained a beachhead on our planet. Some commentators worry that this leaves “only” a few hundred million years for life to emerge—but a hundred million years is a very long time! Asked to speculate on how long it took life to originate, Stanley Miller once suggested that “a decade is probably too short, and so is a century. But ten- or a hundred thousand years seems okay, and if you can’t do it in a million years, you probably can’t do it at all.”
By 3.5 billion years ago, the metabolic diversification that ensured life’s long-term perpetuation had almost certainly begun. Complex microbial communities cycled carbon and other elements through the biosphere. Even photosynthesis may have been present. Earth’s oldest rocks, thus, lie near the juncture where the rootstock of primordial evolution meets the divergence of genes and organisms inferred from the Tree of Life.
Once life got going, where did it lead? By what route did the nascent biology of Warrawoona evolve over three billion years into the animals preserved in Kotuikan limestones? For that, we must resume our historical narrative.
__________
1
Claiming that life on Earth was seeded from Mars (see
chapter 13
), whether probable or not, doesn’t solve the problem; it merely shifts its location.
2
Proteins that catalyze chemical reactions are called
enzymes.
6 | The Oxygen Revolution |
Cherts of the Gunflint Formation, northwestern Ontario, preserve fossils of bacteria that lived nearly 2 billion years ago in an iron-rich sea. Even as the Gunflint rocks formed, however, Earth was completing a major environmental transformation—more than 2 billion years after our planet formed, oxygen began to pervade the atmosphere and surface oceans. The oxygen revolution redirected evolution, ushering in a new biological order that, far in the future, would lead to us.
“T
ake a good chunk. You never know when you’ll be back.” With that admonition, Elso Barghoorn heaved a fifty-pound block of chert into our aluminum dinghy, beached on a rocky promontory along the north shore of Lake Superior (
figure 6.1
).
It is 1974. For Elso, it is the autumn of a patriarch, a nostalgic return to the place where, twenty years earlier, he and Stanley Tyler changed the face of paleontology. For me, the trip provides a first opportunity to see the rocks that inspired me to follow in Elso’s footsteps, a chance to contrast textbook certainty with outcrop reality, and an initiation into paleontology’s oral tradition, passed on by the master as we walk along the shore. The rocks, strewn over our dented boat bottom and exposed in a thin bench along the shore, are Gunflint chert, at 1.9 billion years old nearly as distant in time from Warrawoona as we are from them.
Gunflint begins our return through time to the Cambrian. Do Gunflint fossils extend the enigma of Warrawoona forward or the familiar biology of Spitsbergen backward? As it turns out, they do a bit of both. But more than anything else, Gunflint cherts and associated iron tell us that in middle age, Earth and life were going through important changes.
Figure 6.1.
An outcrop of Gunflint chert along the north shore of Lake Superior. The figure in the distance is Elso Barghoorn, the father of Precambrian paleontology.
The Gunflint Formation is exposed along the lakeshore, in nearby road cuts, and in river-cut gorges of northwestern Ontario. Gunflint rocks include shales, a little bit of carbonate, and here and there some sandstone. A volcanic bed near the top of the formation contains zircons dated at 1,878 ± 2 million years. Most prominently, however, the Gunflint succession features iron formation, the remarkable rock of iron minerals and chert introduced in
chapter 4
. The banded iron formations of Warrawoona and the Barberton Mountain Land are among the oldest examples of this rock type; Gunflint’s are some of the youngest. Iron, thus, provides our first clue to an Earth in transition.
The lowermost unit of the Gunflint Formation contains tiny fossils in fingerlike stromatolites of black chert. That sounds a lot like Spitsbergen, but the apparent similarity is deceptive. Examined closely, all aspects of Gunflint paleontology—fossils, stromatolites, and chert—differ from those of our younger example. In Spitsbergen and most other formations
that contain silica-entombed microfossils, the chert occurs as lenses, nodules or thin beds that formed within carbonate sediments. Gunflint is different. These older cherts formed by silica precipitation directly on the seafloor.