Power, Sex, Suicide: Mitochondria and the Meaning of Life (49 page)
Read Power, Sex, Suicide: Mitochondria and the Meaning of Life Online
Authors: Nick Lane
Tags: #Science, #General
Yes, I hear you say, but we too may live for a hundred years or more; what is so special about a bird that does the same? The answer is that birds live far longer than they ‘ought’ to on the basis of their metabolic rate. If we lived as long as a lowly pigeon, relative to our own metabolic rate, we’d live happily, without much illness, for perhaps a few hundred years. So why not? Why not indeed! Given the political will to overcome the ethical dilemmas, there may be no biological reason why not. Over six million years of evolution, since we split off from the apes, we have already extended our own maximum lifespan by 5- or sixfold, from 20 or 30 years to about 120 years.
1
As depicted in the familiar evolutionary succession, from the stooped knuckle-dragging ape to the erect
homo sapiens
, we have grown in weight as well as stature, and have a lower metabolic
rate. These changes were wrought by natural selection—tampering with the genes—which if we were to apply them to ourselves would be called genetic modification. But even if we lack the stomach to meddle with our genes in the interests of a vainglorious immortality, still the best way to counter the desperate degenerative diseases of old age, which debilitate an ever-growing proportion of the population, is by applying the lessons of evolution in an ethically acceptable way.
I say ‘relative to metabolic rate’. Recall from
Part 4
that in mammals and birds, body mass corresponds to metabolic rate: in general, the larger a species the slower its metabolic rate. For example, the cells of a rat have a metabolic rate that is seven times faster than our own. It’s no coincidence that the rat also lives for a fraction of the time. The relationship between metabolic rate and lifespan can be perceived more directly in insects such as the fruit fly
Drosophila
. In this case, the metabolic rate depends on the ambient temperature, and roughly doubles for every 10°C rise in temperature; and with it, their lifespan falls from a month or more to less than a couple of weeks.
Among the warm-blooded mammals, which are relatively immune to the vicissitudes of weather, there is a broad correlation between body mass, metabolic rate, and lifespan—the larger the animal, the slower the metabolic rate, and again, the longer the life. A similar relationship holds true if we plot out the birds, but now, intriguingly, there is a gap (
Figure 14
). On average, if a bird and a mammal are paired so their
resting
metabolic rate is similar—we might say their
pace of life
is similar—then the bird lives three or four times longer than the mammal. In some cases the discrepancy is even greater. Thus the resting metabolic rate of a pigeon and a rat are similar, yet the pigeon lives for 35 years while the rat lives barely three or four, an order of magnitude difference. We, too, live longer than we ‘should’ if our lifespan is plotted against our metabolic rate—like many birds, and indeed bats, we live three or four times longer than other mammals with similar resting metabolic rates. When I say that we could extend our lifespan to perhaps several hundred years, I am comparing us with the pigeon, which lives two or three times longer than us, relative to its own metabolic rate. Put another way, a pigeon doesn’t live so much longer than a rat because it has slowed down its pace of life. Rather, a pigeon lives ten times longer than a rat while maintaining exactly the same pace of living. There are, apparently, no strings attached.
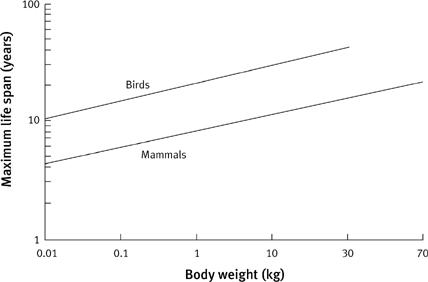
14
Graph showing lifespan against body weight in birds and mammals. Large animals have a slower metabolic rate, and live longer. This is true of both birds and mammals, and the slope of the lines on a log–log plot is very similar. However, there is a gap between the groups: birds live three to four times as long as mammals with a similar body weight and resting metabolic rate.
An important point is that ageing is usually, but not inevitably, linked to disease. The rat suffers similar diseases of old age to us. Rats become obese, get diabetes, cancer, heart disease, blindness, arthritis, stroke, dementia, you name it; but they develop these diseases within a two or three year timeframe, and not over decades. Many birds, too, suffer from equivalent diseases, but always towards the end of their lives. There is unquestionably a link between ageing and degenerative disease, but the nature of this link remains speculative and disputed. There are few things we can say for sure. One is that the link is not chronological—it does not depend on a fixed passage of time, but is relative to the lifespan of the creature concerned. It depends on age, not time; and the rate of ageing is broadly fixed for each species. While there is plenty of variation around the average, it is still not too far from the truth to say, with the bible, that our allotted time in this world is three score years and ten. This allotted time comes from within: it is controlled by our genes in some way, even though it can be modulated to a degree by diet and general health. When asked what finding would make him question his belief in evolution, J. B. S. Haldane answered: ‘a Precambrian rabbit’. Likewise, I will cast all my views on ageing through the window when I meet a centenarian rat. A rat may one day evolve to
live for a hundred years but only after changing a good many of its genes. It will no longer really be a rat.
There is a second point about the link between ageing and disease that is even more pertinent to our own suffering: degenerative disease is not an inevitable aspect of ageing. Some seabirds, for example, seem to sidestep the diseases of old age altogether, and do not age as ‘pathologically’ as ourselves. Like the elves they appear to live long and healthy lives, and somehow avoid many of the afflictions of old age. Exactly what they die
of
is not known with certainty, but it seems the incidence of crash landings rises with age; presumably, despite not succumbing to degenerative diseases, they begin to lose muscle power and coordination. There is a hint that the ‘oldest old’ among humans—those who live well past a hundred—are also less prone to degenerative diseases, and tend to die from muscle wastage rather than any specific illness.
There have been hundreds of theories of why we age. I discussed some of these in
Oxygen
, from a broad evolutionary point of view. Suffice to say here that many of the attributed causes of ageing fall prey to the traps of causality and circular argument. Some say, for example, that ageing is caused by a fall in the circulating levels of a hormone like growth hormone. Perhaps; but
why
do such hormone levels start to fall in the first place? Similarly, others hold that ageing stems from a decline in the function of our immune systems. Certainly this is a factor, but
why
does our immune function start to decline? One answer might be through an accumulation of wear and tear over many years, but this answer, albeit popular, will not do. Why do rats and humans accumulate wear and tear at such different rates? Could not a rat shielded from the slings and arrows of outrageous fortune live to a hundred? Absolutely not! Its rate of ageing is determined from within. We each hold within ourselves a ticking clock, and the speed at which the clock ticks is determined by our genes. In the jargon, ageing is endogenous and progressive: it comes from within, and it gets worse over time. Any explanation must account for these traits.
Most proposed clocks don’t keep good time. The telomeres, for example—the ‘caps’ on the end of chromosomes that wear away over our lives at a steady rate—display such divergent patterns across species that they can’t possibly be the primary cause of ageing. I have already dwelt on the metabolic rate as another clock. This, too, is commonly dismissed as the clock of life on the grounds that the relationship between metabolic rate and ageing can be badly distorted—as in the case of the pigeon with its long lifespan linked to a fast metabolic rate. Unlike the telomeres, however, these distortions offer deep insights into the underlying nature of ageing. The metabolic rate is a proxy, somewhat rough, for the rate of free-radical leakage from the respiratory chains within the mitochondria. Sometimes the rate of free-radical leakage is proportional to the metabolic rate, as among many mammals, but the relationship is
not always consistent: there are many examples where the metabolic rate does not tally with free-radical leakage. Such transgressions potentially explain not just the long lifespan of birds, but also the exercise paradox—the fact that athletes, who consume far more oxygen than couch potatoes, do not age any faster, and indeed often age more slowly.
As an explanation for ageing, free-radical leakage from the mitochondria has been challenged repeatedly, and to convince must overcome a number of apparent paradoxes. Yet overcome them it does. The shape of the mitochondrial theory of ageing has transformed radically since its first exposition, more than thirty years ago. In its latest incarnation, however, it explains not just the broad outlines of ageing, but also many specific aspects such as muscular wastage, persistent inflammation, and degenerative diseases. In the final chapters, we’ll see that the mitochondria are not only the main cause of ageing, but given the traits we have discussed in this book, it is inevitable that they should be. We’ll also see what might be done about it, in our efforts to age with the grace of an elf.
The Mitochondrial Theory of Ageing
Denham Harman, pioneer of free radicals in biology, first proposed the mitochondrial theory of ageing in 1972. Harman’s central point was simple: the mitochondria are the main source of oxygen free radicals in the body. Such free radicals are destructive, and attack the various components of the cell, including the DNA, proteins, lipid membranes, and carbohydrates. Much of this damage could be repaired or replaced in the usual way by the turnover of cell components, but hotspots of damage, most notably the mitochondria themselves, would be harder to protect simply by consuming dietary antioxidants. Thus, spake Harman, the rate of ageing and the onset of degenerative diseases should be determined by the rate of free-radical leakage from mitochondria, combined with the cell’s innate ability to protect against, or repair, the damage.
Harman based his argument on the correlation between metabolic rate and lifespan in mammals. He explicitly labelled the mitochondria the ‘biological clock’. In essence, he said, the faster the metabolic rate, the greater the oxygen consumption, and so the higher the free-radical production. We shall see that this relationship is often true,
but not always
. The proviso may seem trivial, yet it has confounded an entire field for a generation. Harman made a perfectly reasonable assumption, which has proved untrue. Unfortunately, his assumption has become entangled with the theory in general. To disprove it does not disprove Harman’s theory, but it does overturn his single most important, and best-known, prediction—that antioxidants can prolong life.
Harman’s sensible but confounding assumption was that the
proportion
of free radicals that leak from the mitochondrial respiratory chains is constant. He assumed that the leakage is basically an uncontrolled, unavoidable by-product of the mechanism of cell respiration, which juxtaposes the passage of electrons down the respiratory chains with a requirement for molecular oxygen. Inevitably, the theory goes, a proportion of these electrons escape to react directly with oxygen to form destructive free radicals. If free radicals leak at a fixed rate, let’s say 1 per cent of total throughput, then the total leakage depends on the rate of oxygen consumption. The higher the metabolic rate, the faster the flux of
electrons and oxygen, and the faster the free-radical leakage, even if the proportion of free radicals actually leaking never changes. So animals with a fast metabolic rate produce free radicals fast and have short lives, whereas animals with a slow metabolic rate produce free radicals slowly, and live a long time.
In
Part 4
, we saw that the metabolic rate of a species depends on its body mass to the power of 2/3: the larger the mass, the slower the metabolic rate in individual cells. This link is largely independent of the genes, depending instead on the power laws of biology. Now, if free-radical leakage depends
only
on the metabolic rate, then it follows that the only way to prolong the lifespan of a species
relative to metabolic rate
, is to bolster the strength of antioxidant (or anti-stress) protection. An implicit prediction of the original mitochondrial theory of ageing is therefore that creatures living a long time must have inherently better antioxidant protection. Birds, then, which live a long time, must stockpile more antioxidants. Accordingly, if we wish to live longer ourselves, we must seek to bolster our own antioxidant protection. Harman supposed that the only reason we have failed to extend our own lifespan by taking antioxidant therapy so far (this back in 1972) is because it is difficult to target antioxidants to the mitochondria. Many people still agree with this position today, despite another thirty-plus years of industrious failure.
These ideas are tenacious but erroneous, in my opinion: they cling to the mitochondrial theory of ageing like burrs. In particular, the idea that antioxidants might prolong our lives is the basis of a multibillion-dollar supplement industry, which has remarkably little solid evidence to support its claims; unlike the biblical house that was built on shifting sands, it has somehow remained standing. For over thirty years, medical researchers and gerontologists (including myself) have flung antioxidants at all kinds of failing biological systems and found that they just don’t work. They may correct dietary deficiencies, and perhaps protect against certain diseases, but they don’t affect maximal lifespan at all.