Stories in Stone (35 page)
Authors: David B. Williams
Despite the numerous benefit of pens over chalk, whiteboards are far from ideal.
Chalk dust can be an irritant to some people,
but generally the large particles settle out of your nose before they have a chance to get into your lungs; whiteboard cleaners,
though, produce toxic fumes.
The cleaners come with warning labels.
Another drawback to whiteboards is the residue of writing
that doesn’t wash off, no matter how much cleaner you use.
And when someone uses a Sharpie pen by accident, you cannot erase
it from a whiteboard.
Furthermore, half the time you try to use dry-erase pens they don’t work because they have run out of
ink.
Then what do you do?
Toss them in the garbage so they can end up as landfill.
No one ever has to doubt whether a piece
of chalk is usable, and when it becomes too small to write with, you can just carry it outside and bury it.
It will soon degrade
and disappear without a trace.
As I wandered the hallways at Stevens, I couldn’t help but wonder if today’s students are missing out.
Where once they could
have continued the centuries-old tradition of employing fossil sea critters to write or draw on a metamorphosed slab of fine-grained
marine sediment, now they write on petroleum-based, plastic whiteboards with an odoriferous, chemical-filled pen.
In a society
where our failed connection to nature surely has contributed to our failed understanding of human impact on the land, the
loss of slate and chalk is one more example of how we are taking nature away from children and replacing it with something
artificial.
Perhaps this connection was the most utilitarian aspect of slate and now it, too, is lost.
“A
UTUMN
20,000 Y
EARS
A
GO”—
I
TALIAN
T
RAVERTINE
After they had noted what a profusion of resources has been begotten by
Nature, and what abundant supplies for construction have been prepared
by her, they nourished these with cultivation and increased them by means
of skill and enhanced the elegance of their life with aesthetic delights.
—Vitruvius,
Ten Books on Architecture
, book two
He’ll study weird stuff that grew in his sink last week, for instance,
bird droppings, a bit of arterial plaque, or his wife’s cataract.
His instincts
are amazing, though.
No matter how oddball, the things Dr.
Folk chooses
to look at often end up teaching us something about rocks.
—Dr.
Kitty Milliken, University of Texas
G
EOLOGISTS DO NOT normally receive standing ovations when they make presentations at meetings.
Typically, attendees clap politely,
ask a few questions, and then the next presenter walks to the podium and begins his or her talk.
In October 1992, however,
geologist Robert Folk received a standing ovation for his fifteen-minute presentation at the annual Geological Society of
America meeting in Cincinnati.
He had titled his talk “Bacteria and Nannobacteria Revealed in Hardgrounds, Calcite Cements,
Native Sulfur, Sulfide Minerals, and (yes) Travertines.”
Folk’s talk focused on his work from calcite-rich rocks in Italy, as well as the Bahamas, Utah, and Florida.
He began by describing
how he had used hydrochloric acid to etch, or eat away, surface material to reveal undisturbed layers of calcite.
With this
novel etching technique, Folk reported that he had been able to exhume microscopic (requiring greater than 20,000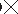 magnification) bacterial bodies from the rocks, primarily from a type of limestone called travertine, and that the microbes
magnification) bacterial bodies from the rocks, primarily from a type of limestone called travertine, and that the microbes
had “emerged from the calcite like cadavers on Judgment Day.”
He called his microbes “nannobacteria,” in reference to their nanometer-scale size.
(Folk prefers his double-
n
construction, but most nongeologists prefer “nanobacteria” or the less controversial—read as no indication of life—“calcifying
nanoparticles.” Folk’s use of “nannobacteria” still rankles many biologists.) No one had ever reported and shown photographs
of such microscopic organisms.
Prior to Folk’s work, the smallest recorded bacteria stretched 200 nanometers in diameter,
or .005 the diameter of the proverbial head of a pin.
In contrast, some of Folk’s nannobacteria were as small as 25 nanometers
or .001 the volume of previously described bacteria, although many ranged up to 150 nanometers.
The bacterial bodies resembled
“ears of corn” and had “great fossilization potential,” he concluded.
Chris Romanek was one of those who attended Folk’s 1992 talk.
Now a geologist at the University of Georgia, Romanek was then
a postdoctoral fellow at NASA.
“I had sort of wandered in, not knowing who was speaking,” said Romanek.
“I remember he was
talking about using an SEM [scanning electron microscope] to see microorganisms in these travertines from Italy.
I thought
this is kind of interesting.”
1
When Romanek finally learned who was speaking, he knew at once who Folk was: “I had used his textbook in college.”
First published in 1974,
Petrology of Sedimentary Rocks
has long been a bible to geologists studying the rocks that make up 80 percent of Earth’s crust, including limestone, sandstone,
and coquina.
Out of print for years, it is now available online from the University of Texas in Austin, where Folk taught
geology from 1952 until his retirement in 1990.
Folk wrote the book to supplement lectures and labs that he gave in Austin
and based it on lectures he had attended at Pennsylvania State College, where he received his bachelor’s, master’s, and doctoral
degrees.
The book is so legendary that geologists have been known to come up to Folk at geology meetings and ask him to autograph
their dog-eared copies.
“Folk’s talk got me thinking about limestones and how they would be conducive for trapping and fossilizing microbes,” said
Romanek.
Although his specialty was carbonate rocks such as limestone that contain the minerals calcite, dolomite, and aragonite,
he had not thought about this connection between physical and biological processes in their formation.
It was a connection
that would have profound implications for Romanek’s research.
Thirteen months later, Folk’s talk came back to Romanek when his colleague at NASA, David Mittlefehldt, asked him to look
at pictures of orange blobs on a meteorite from Mars.
Mittlefehldt told Romanek that the microscopic, pumpkin-colored rosettes
were carbonates.
The photographs stunned Romanek, who had never seen carbonate minerals on a rock from outer space.
He immediately
asked Mittlefehldt if he could get a sample from the rock, which had been found in Antarctica in 1984.
Romanek believed he
had the tools, primarily through Folk’s etching technique, to tease out the answer to the underlying question of how carbonates—minerals
often associated with water and living organisms—developed on a potato-sized rock that had traveled millions of miles across
space.
After receiving a pinhead-sized chip of the Martian meteorite, Romanek etched his sample with acid and began to probe the
rock.
Looking under the SEM, Romanek found a screen filled with bacteria.
He couldn’t believe what he was seeing, life forms
on a rock from outer space.
Unfortunately, he wasn’t seeing microscopic ETs; instead, Romanek was looking at Texas bacteria.
The microbes that polluted his sample came from unfiltered water he had used to dilute his etching acid.
He tried again, this
time with filtered water, and again saw features that looked like relicts of bacterial organisms through the SEM.
The rod-and
ball-shaped structures resembled the nannobacteria Folk had described from travertine at his 1992 Cincinnati talk.
Romanek knew that he had found something extraordinary within the meteorite’s orange blobs.
Since the rods and balls were
discovered within carbonates—minerals known to form via biologically induced deposition—the structures could be organic remains.
For the next two years, Romanek and his fellow researchers at NASA probed, lasered, and scoped the meteorite to determine
whether Romanek’s initial observations were correct.
They also reviewed Robert Folk’s extensive work on Italian travertine,
in particular a paper he published in 1993 that showed two dozen photographs of nannobacteria.
On August 7, 1996, NASA astounded the world when they reported what Romanek and his colleagues had discovered in their Martian
meteorite from Antarctica.
The 4.5-billion-year-old rock, known as Alan Hills 84001 (ALH84001), contained evidence for life
on Mars.
Chris Romanek’s observations on nannobacteria-generated carbonates were central to NASA’s argument.
“If I had not
seen Bob Folk’s talk, I wouldn’t have thought to do what I did,” said Romanek.
“His work definitely kick-started the whole
NASA project.” And Folk’s work all started with travertine, a building stone quarried in Italy for more than two thousand
years.
Roman architect and writer Marcus Vitruvius Pollio may never have given a fifteen-minute-long talk at an annual meeting, but
like Robert Folk, Vitruvius was a close observer of Italy’s sedimentary rocks.
In his landmark
De Architectura
, or
Ten Books on Architecture
, he devoted his second book to building materials.
“I thought that I should expand on their varieties and the criteria for
their use, as well as what qualities they have in building, so that when this information is known, those who are planning
to build will avoid mistakes and assemble supplies suitable for buildings,” wrote Vitruvius sometime between 31 and 27 BCE.
2
Vitruvius based his work on empirical investigations he made of the building stones quarried near Rome.
In the category of
soft and yielding rocks, he included the olive gray
Tufo Lionato
erupted from the Al-ban Hills southeast of Rome, and
Tufo Giallo della Via Tiberina
(yellow tuff from the Tiber Road), erupted from Monte Sabatini, north of Rome.
Tuff is rock composed of volcanic glass fragments,
crystals, and rock fragments deposited by pyroclastic flows produced by violent eruptions of magma.
In Rome tuff forms resistant
layers in the city’s celebrated seven hills, one of which—Palatine—provided a source for building material in the fifth and
sixth centuries BCE.
Conversely,Vitruvius’s hard, enduring stones correspond to lava flows that crop out near Rome.
Roman builders often used these
lavas for paving stones.
They still pave many streets in the Eternal City.
Locals call the three-inch-square paving blocks
San Pietrini,
“little Saint Peters,” playing on St.
Peter’s role as the rock of Christianity.
Vitruvius also described rocks of moderate
durability, including travertine.
Travertine is a sedimentary rock rich in calcite and the related mineral aragonite.
Layered, dense, and well-cemented, travertine
forms primarily in hot springs.
The Romans used it as early as the second century BCE for voussoirs to strengthen the
Pons Mulvius
, which spanned the Tiber.
They quarried most of their travertine in Tibur, now Tivoli, about twenty miles east of Rome.
During
Vitruvius’s day, travertine was known as
Lapis Tiburtinus
, “stone of Tibur,” later shortened to
tiburtino
and corrupted to
travertino
, travertine in English.
Modern quarries at Tivoli supply most of the travertine used in the building trade; it is the most
commonly used and commercially valuable building stone in the world.
Although the Roman tuffs had the virtue of being easily worked, Vitruvius wrote that “if they are put in open, uncovered places,
then once they have been saturated with ice and frost they crumble apart and dissolve.
Likewise, along the seashore they will
wear away, eaten by the salt.” In contrast, travertine “endure[s] every strain, whether it be stress or the injuries inflicted
by harsh weather.” To deal with the weaker tuffs, Vitruvius recommended builders quarry the stone in summer, let it sit in
the open for two years, use only the best, and dump the crappy stone in with foundations.
Builders could further protect tuff
from water by covering blocks with plaster or by placing travertine on top of the blocks.
The Theater of Marcellus, built in 13 BCE, exemplifies the building techniques described by Vitruvius, according to Marie
Jackson, a geologist who has written extensively on the technical expertise of Roman builders.
3
For the three-story structure, the Romans selected travertine for exterior arches and carved columns.
The stone is scarred
and cracked but still retains a sturdy grandeur.
For the interior wall, the builders used brown tuff for the arch shafts,
but in locations that had to withstand the greatest stresses, such as imposts and keystones, they chose travertine.
To further
protect the tuff, builders most likely applied thick stucco, which has long since eroded away.
Less famous than the more centrally
located and more imposing Colosseum, the Theater of Marcellus epitomizes a sense of the majesty of ancient Roman construction,
the technical mastery of the engineers, and the beauty of its building stones.
Travertine building blocks, though, have a weakness.
“They cannot be safeguarded against fire.
As soon as they make contact
with it, they crack apart and fall to pieces,” wrote Vitruvius.
(Like the Greek philosophers Aristotle and Plato, Vitruvius
considered that matter consisted of unique combinations of the elements water, earth, air, and fire, which gave an object,
such as a stone, unique properties.
Too much air and fire, according to Vitruvius, made travertine susceptible to breaking
at high temperatures.) Modern scientists point to the unequal amounts of extension and contraction along internal crystallographic
axes in calcite for travertine’s poor performance in fires.
Jackson found, however, that tuff survives fire better than travertine
because of its porous texture, which allows tuff to expand when heated with far less fracturing than travertine.