Why Is Milk White? (28 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho
Pharmacists are not just concerned with how their chemicals affect the body of the patient, but they are also concerned with how the molecules interact with one another, how they affect the body in combination, and how they are delivered. To make a pill that survives the stomach acid and is delivered into the intestines can be complicated.
Some drugs must be injected or delivered through the skin in some other way, because they can't pass through the digestive tract unaltered. Most proteins are in this category. Some are inhaled for the same reason or because they act faster that way. Knowing how chemistry affects drug delivery is an important part of designing pills, capsules, injectables, and inhalants.
HOLLOW PENNIES
Materials
Stack of pennies minted after 1982
Metal file, nail file, or emery board
2 teaspoons salt
½ cup vinegar
Water
Thread or wire, long enough to make a necklace
Muriatic acid (optional, available at hardware stores and swimming pool supply houses)
Protective goggles (optional)

In 1982 the US Mint stopped using 95 percent copper for pennies and switched to a cheaper design, made of zinc coated with a thin layer of copper.
In the photo on the right I have a stack of pennies from after 1982, each of which has had a bit of the copper plating filed off the edges. I filed each penny on opposite sides, so the zinc shows through on the top and the bottom edges. If I could remove the zinc from inside, leaving only the paper-thin copper, I could string these hollow penny shells on a string to make a necklace.
As it turns out, you can. Many acids will react with zinc but not with copper. One such acid is hydrochloric acid. You can make a weak (and safe) form by combining about ½ cup
of vinegar with about 2 teaspoons of salt (as much as will dissolve in the vinegar). You'll have to wait a day or two for it to do the trick, though. For a much faster reaction, you can use a stronger form of hydrochloric acid called muriatic acid, available at hardware stores and swimming pool supply houses. When handling muriatic acid,
always use eye protection,
and make sure you have
adult supervision.

Of course, I chose the faster method. Here you can see hydrogen bubbling up from the filed pennies as the exposed zinc reacts with the acid.

The acid also cleans off any tarnish from the copper. As it reacts with the tarnish, more hydrogen is released, and you can see that while most of the bubbles are coming from the filed-off places, there is still some coming from the rest of the penny as the tarnish reacts with the acid.
After a few hours, the pennies will float to the top of the acid
and stop bubbling. They float because they are thin copper shells of their former selves and they are filled with hydrogen.

Now you can add a lot of water to the jar to dilute the acid, and it can be safely poured down the sink. Rinse the pennies well, and then you can string them on a thread or a wire to make a necklace.
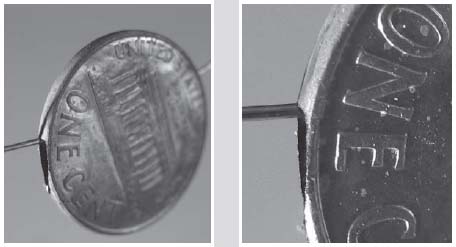
A close-up view shows the penny is actually hollow.
Many drugs act on the body in similar ways, and when used in combination, they can have harmful effects. Pills that make you drowsy are generally not safe to take when drinking alcohol, for example. The alcohol can increase the effects or side effects of the drug, and the drug can increase the effects of the alcohol.
In other cases, a drug might cause the body to produce an enzyme that breaks down another drug the patient might be taking. Understanding chemistry allows the pharmacist to suggest that the pills be taken at different times or that another drug be substituted for one of the originals.
Your doctor knows a surprising amount of chemistry. The chemistry of blood or urine or even of your breath can all tell her something about your health. She may suggest chemicals to alter some chemical process in your body, such as statin drugs to lower your cholesterol, or vitamins to make your body function properly.
Your body is a vast chemical processing plant, and much of what medicine is about is concerned with how the body produces and uses chemicals. Even when the doctor is just sewing up a cut on a finger, she might be thinking about the chemistry of blood coagulation, the brain chemistry of pain, and how aspirin might affect both of them.
Doctors use chemistry to control infections, both by washing themselves, their tools, and their patients with disinfectants and by giving the patient chemicals that attack bacteria without harming the patient's tissues.
The adhesive on a Band-Aid was designed by a chemist. So was the plastic it was made from.
A doctor might need to recognize the symptoms of poisoning caused by chemical exposure or drug overdoses. Even more
understanding of chemistry helps her repair or mitigate the damage by absorbing or neutralizing the poison or by helping the body heal afterward.
One of the doctors most concerned with chemistry is the anesthesiologist, the doctor who controls your state of consciousness during surgery. He needs to know the effects of oxygen and the many drugs at his disposal and how to monitor the patient for signs of trouble.
All of the ingredients used in the kitchen are chemicals, and almost all of the techniques used in cooking are concerned with chemical changes in the food you prepare.
The baking powder you use to make pancakes rise is a good example. Sodium bicarbonate powder supplies the carbon dioxide bubbles, and dry acid salts such as tartaric acid and monocalcium phosphate are used to release it by reacting with the bicarbonate. These reactions take place as soon as water is added to the dry powder. Other acid salts such as sodium aluminum sulfate are also added, since these only react at higher temperatures in the oven or on the griddle. This allows the batter to still rise after it has been sitting on the counter for a while.
A chemist knows that green vegetables turn an ugly drab color when the magnesium atom at the center of the chlorophyll molecule is replaced by a hydrogen atom. This can happen when green vegetables are heated or when an acid is present. Cooking for a shorter time or avoiding acids like vinegar or lemon juice can keep the colors bright.
Knowing what temperatures change the structure of the proteins in food can also be extremely helpful in cooking. Each of the proteins in an egg, for example, hardens at a different temperature. If you can keep your egg at a temperature at which all of the white hardens but little or none of the yolk does, you can have very tight control over how your eggs are cooked.
Controlling the temperature while meat cooks is important for the same reason. If you never allow your meat to reach the temperature at which the meat proteins harden, you can avoid a tough cut, while allowing a high enough temperature to convert all of the tough connective tissue into soft gelatin.

Everyone loves to eat, and
needs
to eat. Our bodies are made of chemicals, and we need new chemicals to fuel us, build the parts that grow, and rebuild the parts that are constantly wearing out. Chemicals are what make food look, smell, and taste the way they do. They are what make food healthy and good for us. We tend to forget about all the good chemicals in food because we fear the ones that are bad for us or that we don't understand. But knowledge of food chemistry can alleviate that fear.
Of course all food is made from chemicals. But this book has discussed the meaning of the word
chemical
earlier and found that people without a scientific education confuse the word
chemical
with concepts such as things in my oatmeal you can't pronounce.
Oatmeal is a wonderful food all by itself. The Fruit & Maple Oatmeal you get at McDonald's might have far too much sugar added, but that is not the “chemical” that people are complaining about, even if it is the most dangerous ingredient in the bowl.
The oatmeal contains rolled oats, brown sugar, modified food starch (regular starch broken down into smaller molecules to change its thickness), salt, natural flavor (from plant sourcesâspecifically the maple flavor), barley malt extract (what you put in malted milk), and caramel color (burned sugar).