A Brief History of Creation (37 page)
Read A Brief History of Creation Online
Authors: Bill Mesler
Linnaeus's classification scheme was based on fairly easily observable traits: whether things moved or grew, swam or flew, had fur or backbones. He grouped things by physical similarity. Fossils would eventually provide meaning to the scheme, suggesting an evolutionary pattern that linked the different species and providing a body of evidence upon which Darwin's theory of natural selection would rest.
Darwin was among the first to envision a phylogenetic “tree of life,” a family tree that stretched back through every generation to the beginnings of life. He included a sparse and simple drawing of his tree in
On the Origin of the Species
. It consisted of a twig-like branching pattern, with extant species represented by the outermost twig points. According to Darwin's scheme, tracing downward from these points would be like traveling back in time, with junctions in thicker and thicker branches where each twig would have a common ancestor. Humans and chimpanzees, for example, would have diverged from a common ancestor, and if one followed the branch leading to that junction backward, it would eventually join at another juncture with yet another branch leading upward to, say, New World monkeys. One could also travel down the tree through evolutionary timeâthrough the divisions of mammals, the vertebrates, the animalsâwith the tree gradually narrowing until ending at the single organism that is the root of all existing life. Darwin logically concluded that
all
living things must have been descended from one common ancestor, what he described as “one primordial form.”
The notion of universal common ancestry became a central tenet of modern evolutionary theory. It was supported by a number of observations, like chirality (first discovered by Pasteur in his work with crystals), the similarities of cellular structures, and the fact that every organism, from microbes to human beings, uses almost exactly the same genetic
code. By the modern era, few reputable scientists would argue against the doctrine of common descent.
In time, Darwin's tree of life was enhanced and reshaped by modern paleontology and radiometric dating. Bones could be dated, and the family lineages of developed species could be more accurately reconstructed. As techniques in microbiology improved, organisms were further divided into single-celled and multicellular organisms, and later into two different categories: organisms with a cell nucleus, called the eukaryotes, and those without, the prokaryotes. Eventually, the living world would be divided into five kingdoms: animals, plants, fungi, single-celled eukaryotes, and prokaryotes. But the pool of evidence for the last two kingdoms was lacking. The fossil record of the most numerous, simplest, and presumably oldest species was glaringly thin, and the place of microbes in the tree of life was shaky. Carl Woese was determined to find a way around that problem.
I
N 1969, WOESE WROTE
a remarkable letter to Francis Crick. It turned out to be a kind of blueprint for how he would spend the next twenty years, and what he hoped toâand eventually wouldâaccomplish. Woese told Crick that he planned to use DNA to reveal what he called the cell's “internal fossil record,” establishing the true relationships between organisms: “By deducing rather ancient ancestor sequences for these genes, one will eventually be in the position of being able to see features of the cell's evolution.” He had recognized the potential for molecular biology to use the genetic code to fill in the early gaps in evolution that the fossil record couldn't complete. He planned to do this by sequencing a gene that was common to almost every living thing and then using that gene to trace the history of evolution.
By the early 1960s, the process of sequencing the amino acids of proteins had become routine. Emile Zuckerkandl and Linus Pauling took proteins from modern organisms that could already be placed in the phylogenetic tree and showed that the sequences of the proteins differed depending on how recently the species had diverged according to the fossil record. By measuring the differences between proteins from different organismsâwhat
they called a “molecular clock”âthey could calculate just how long ago organisms had diverged during their evolutionary history.
But not all proteins are common to every species. Woese needed to work with something that was present in
all
known organisms, was copied with a great deal of precision, and mutated slowly enough that it could be easily tracked over billions of years. He settled on genes for a type of ribosomal RNA called 16S, so named after the rate at which it sediments in a centrifuge. The 16S RNA genes were long enough to yield detailed data but short enough that they wouldn't be too difficult to sequence.
By the time Woese began his gene sequencing in earnest, he had moved on from General Electric to the University of Illinois at Urbana-Champaign, recruited for a faculty position by the molecular biologist Sol Spiegelman, who had attended a talk that Woese delivered at the Pasteur Institute in Paris. At Illinois, Woese gathered a small team, the most prominent of which was his postdoctoral assistant, George Fox, who would go on to share credit for the team's most important discovery. Together, they began the complicated process of sequencing the genes for 16S ribosomal RNA, or “16S rRNA” for short.
All the analyses had to be done by hand; automated sequencing was still decades away. Woese and his team adopted a technique that had been developed in 1965 by British biochemist Fred Sanger, one of the rare people to have won two Nobel Prizes. Sanger's process involved dividing the genes for the RNA into small, workable pieces by cutting them with enzymes. The small pieces could then be sequenced, after which the whole molecule would be reassembled and the full genetic sequence revealed. Such work was expensive, and Woese turned to NASA exobiology grants to help fund the research. The work was also painstakingly slow. At the start of the project, sequencing a single 16S rRNA gene could take months. For most scientists, such work would have been tedious in the extreme, but Woese relished it. It was like putting together an enormous jigsaw puzzle.
By the spring of 1976, Woese's team had accumulated complete 16S rRNA sequences from a large variety of bacteria. He and his team then turned their attention to a unique set of microbes known as methanogens.
Unusual microbes to the extreme, methanogens had been named for their strange ability to produce methane as a by-product of metabolizing carbon dioxide and molecular hydrogen for energy. Because of their observable, physical characteristics, scientists had long assumed that methanogens were a form of bacteria, but Woese's genetic data established that they were not bacteria at all. He realized that he and his colleagues had upset the foundation of biological taxonomy. Instead of two ancient lineagesâeukaryotes and prokaryotesâthere were three, all of which had branched off separately soon after the beginning of life.
Woese called his new family the “archaebacteria” but later shortened the name to simply “archaea,” meaning “the ancient ones.” He then began redrawing the tree of life. In Woese's tree, everything that would have made up Darwin's tree could be grouped into a mere twig at the end of a single branch. By the time Woese was finished, the tree looked more like a complex, asymmetric snowflake with three distinct branches going in different directions from the base. He called these three main divergences “domains.” The discovery came to be known in microbiology circles as the “Woesian revolution.”
In 1977, Woese, Fox, and NASA jointly announced the discovery of the archaea in a press release that coincided with the publication of a scientific paper in the
Proceedings of the National Academy of Sciences
. The discovery was greeted with skepticism, incredulity, and, at times, anger. It didn't help that Woese had already developed a reputation with some scientists as an odd recluse who was working on an impossible question. Some saw his data as too fragmented to redraw the map of evolutionary relationships. A few thought he was a little crazy. One of the most influential evolutionary biologists of the twentieth century, the German-American biologist Ernst Mayr, became Woese's most ardent critic, telling the
New York Times
that Woese's work was sheer nonsense. Woese's preferred form of defense against critics was to send repeated letters to the editor, which did little to help his cause. When the first major scientific conference was held to discuss his theories, he was not even invited. He might not have attended even if he had been.

Woese holds a model of RNA in 1961.
By the mid-1980s, Woese's views had nevertheless gained some acceptance. In 1992, he was awarded microbiology's most prestigious award, the Leeuwenhoek Medal of the Royal Netherlands Academy of Arts and Sciences, conferred once every ten years. In 1996, Woese, his fellow University of Illinois professor Gary Olsen, and their teams published the first complete genome structure of an archaean,
Methanococcus jannaschii
. In an article in
Science
, they concluded that the archaea are more closely related to humans than to bacteria. “The archaea are related to us, to the eukaryotes,” Woese said in an interview shortly after the discovery. “They are descendants of the microorganisms that gave rise to the eukaryotic cell billions of years ago.”
Woese's work on
Methanococcus jannaschii
turned the tide of scientific opinion heavily in his favor. The new taxonomy was adopted by most
scientists. Reflecting on his dramatic vindication,
Science
dubbed Woese “Microbiology's Scarred Revolutionary.” The moniker stuck with him for the rest of his life.
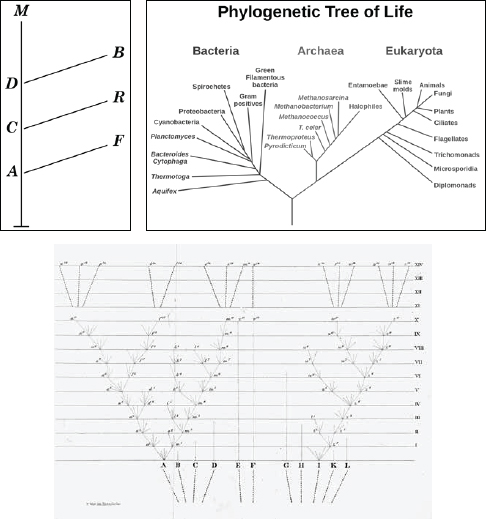
The Tree of Life according to Chambers (top left), Woese (top right), and Darwin (bottom).
T
HE WOESIAN REVOLUTION
had a profound impact on the study of the origin of life. Before Woese, the question of origin was approached mainly from the bottom up. Scientists like Stanley Miller and Sidney Fox had searched
for the earliest chemical processes that led, step by step, to the creation of the first living organism, by starting with the earliest steps that must have been necessary for life to appear.
Woese strengthened the case for approaching the question from the opposite direction. The history of life could be traced backward using the evolutionary evidence that still existed in the genes of modern organisms, each of which had evolved from a common ancestor. Tracing the genetic records of different species made it possible to obtain a picture of the earliest ancestor. The characteristics shared by the clusters of microorganisms that made up the bottom of the tree were likely traits they held in common with LUCA. And those traits could show not only what LUCA looked like, but what the world that LUCA was adapted to live in looked like. It was still a hazy picture, but it was far less hazy than it had been before.
One of the problems, though, was the unique manner in which the oldest life-forms had shared their genetic information. This unusual way of sharing genes would lead Woese to make one of his most daring and controversial assertions, that the first life was not an organism at all, but rather a community of organisms that freely swapped information in a collective genetic pool through a process called “horizontal gene transfer.” It was an idea that sprang from Woese's attempts at unlocking the genetic code.
Woese was fascinated by the code's resistance to change over billions of years, and through millions of distinct evolutionary branches. With a few very minor exceptions, every modern blue whale, redwood tree, human, and microbe speaks an identical genetic languageâremarkable, considering that human languages, over remarkably short timescales, tend to drift into dialects. The genetic code has barely changed at all for billions of years. The reason for its durability remained an enigma as scientists first began to confront the problem in the 1960s.
Woese saw a clue in a discovery that had been made in 1951 by a Seattle physician named Victor Freeman. Freeman discovered that genes from a virus that infects a disease-causing strain of the bacterium
Corynebacterium diphtheriae
could be used to transform a harmless bacterial strain into a virulent one. This finding explained why people infected with diphtheria sometimes don't get sick immediately but often succumb to
symptoms later. Perhaps more important, Freeman's observations of
Corynebacterium diphtheriae
documented one of the first known examples of horizontal gene transfer, the swapping of genes between distinct organisms. Eventually, manyâperhaps even mostâmicroorganisms would be found to use horizontal gene transfer. Woese hypothesized that this mode of genetic exchange explained why the genetic code had not changed in billions of years. Organisms needed a stable, uniform language with which they could genetically “speak” to each other. If their genetic code changed, the beneficial trait of being able to swap genetic information with other organisms would be lost.