A Brief History of Creation (39 page)
Read A Brief History of Creation Online
Authors: Bill Mesler
Cech and Altman's discoveries revolutionized our understanding of biochemistry. Some proteins catalyze chemical reactions. Some are motors. Still others, embedded in cell membranes, open and close channels that make it possible for the phenomena of consciousness to take place. Eating, digesting, moving, even thinkingâall are, at their most basic level, functions of proteins. Proteins control the chemical processes responsible for virtually everything an organism does at the cellular level, and proteins had always been understood to be the sole cellular components carrying out these reactions. But Cech and Altman proved that some RNA moleculesâwhich came to be called ribozymesâcould also act as catalysts like proteins do. The discovery earned each a share of the 1986 Nobel Prize in Medicine.
As Gilbert pointed out in his
Nature
article, the discovery of ribozymes had huge implications for the study of the origin of life. Here was a part of the cell that could accomplish
both
replication and metabolism. He argued that at some point very early in the history of evolution, the simple cells that populated the Earth merely contained RNA. “The first stage of evolution proceeds,” he wrote, “by RNA molecules performing the catalytic activities necessary to assemble themselves . . . [eventually] using recombination
and mutation to explore new functions to adapt to new niches.” The theory came to be known by the phrase with which Gilbert had titled his influential article, “The RNA World.”
B
OTH CECH AND ALTMAN
had come to their groundbreaking revelations about RNA while working with samples taken from a microscopic protozoan called
Tetrahymena thermophila
.
Tetrahymena
is a remarkable little creature, part of the ciliate family, eukaryotes first observed by Antonie van Leeuwenhoek and characterized by the stringy cilia that spring from them like clumps of hair.
Tetrahymena
comes in seven different “sexes” and is capable of thriving over a wide range of temperatures. But what particularly sets it apart from other single-celled organisms is the wide variety of biological processes it shares with more complex organisms. It has a primitive digestive system, with a mouth-like pore for ingesting food. Remarkably, the tiny organism contains about twenty-five thousand genes, nearly as many as human beings have. The presence of such a wide array of
genes makes
Tetrahymena
useful for modern biological research, as does the ease with which it can be cultured.
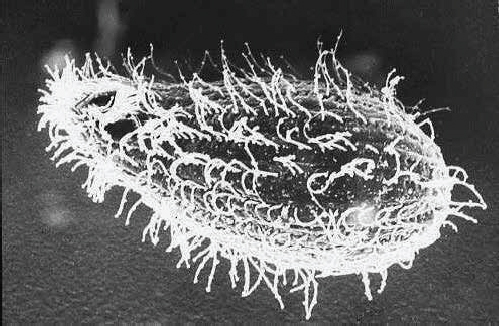
A Tetrahymena as seen by a scanning electron micrograph.
Tetrahymena
had already been at the center of an absurdly large number of important biological discoveries, including the identification of the first cytoskeleton-based motor protein (a primitive muscle-like protein) and the existence of lysosomes and peroxisomes, which are like the cell's little wastebins. In fact, the very same sample of
Tetrahymena
used by Cech and Altman was also being used by a completely different group of researchers who would go on to win a Nobel Prize for a completely different discovery. One of those scientists, a young, mild-mannered Canadian named Jack Szostak, would go on to build upon the discovery of ribozymes to become one of the leading origin-of-life scientists of the twenty-first century, and the most famous of the modern scientists who actively study the RNA-world model.
T
HE SON OF A PILOT
in the Royal Canadian Air Force, Szostak was captivated by the
Apollo
missions during his childhood. But he had always been more interested in the experiments the astronauts would conduct on the moon than in space travel itself. Biology had been a particular area of interest. In school, he was a prodigy. He enrolled in McGill, Canada's most prestigious university, when he was just fifteen years old.
After joining Harvard Medical School as a professor of chemistry in 1982, at the age of twenty-seven, Szostak first turned to the subject of DNA repair in the yeast
Saccharomyces cerevisiae
, a model eukaryotic organism that had fascinated countless scientists since Pasteur's time. Soon thereafter, he attended a lecture given by UC Berkeley molecular biologist Elizabeth Blackburn regarding the genetics of
Tetrahymena
. Szostak felt Blackburn's advances could be brought to bear on work he was doing in his own lab that involved a long-standing problem in eukaryotic cell biology: Because the enzymes that copy DNA never quite reach the ends of chromosomes, scientists had always expected that part of the chromosome should remain uncopied at the end of each round of cell division. That this was not always true had long left scientists puzzled.
Along with molecular biologist Carol Greider, Szostak and Blackburn
devised a set of experiments to figure out what was happening. By making
Saccharomyces
-
Tetrahymena
hybrid chromosomes, they were able to show that tiny pieces of DNA called telomeres, Greek for “end part,” from the ends of
Tetrahymena
chromosomes could protect
Saccharomyces
chromosomes from being shortened, and vice versa. This finding not only explained the paradox; it had significant implications for understanding cellular aging and cancer. Szostak, Blackburn, and Greider's work was published in 1982. In 2009, nearly three decades later, the three shared a Nobel Prize for the discovery.
A
FTER HIS WORK WITH
telomeres, Szostak began looking for a new scientific challenge. Very early in his career, he had decided he wanted to work on what he considered the three big questions in science: the origin of the universe, the origin of consciousness, and the origin of life. He realized early on that his math wasn't up to par to tackle the physics associated with the origin of the universe. And though, like Henry Bastian and Francis Crick, he was tremendously enticed by the idea of unraveling the phenomenon of consciousness, he felt the technology wasn't yet there to make much of an impact. But after Cech and Altman's discoveries about the nature of RNA, Szostak saw an opportunity to make important strides in understanding the origin of life.
As early as 1984, Szostak had started to immerse himself in the study of ribozymes, trying to better understand what their roles might have been in the earliest cells. His goal switched to finding something that was held up as a kind of holy grail among those who subscribed to the RNA-world theory: an RNA molecule that could do the work of copying itself. Nothing like it existed, or had ever existed, as far as anyone could prove. But Szostak had new avenues to approach the problem that hadn't been available to many of his predecessors.
Since the Miller-Urey experiment, re-creating the chemical steps that would have led to FLO's initial appearance had proved perplexingly difficult. Though some progress had been made, few scientists had had much success. But Szostak recognized that there might be another way of approaching the problem. By the 1990s, with advances in knowledge about the cell and the development of modern techniques to manipulate the cellular
machinery, it became possible for scientists to consider building a cell from scratch. Instead of trying to re-create all the difficult chemical steps necessary for the emergence of the first life, Szostak was simply trying to create it in his laboratory.
T
HE ERA OF
completely synthetic life-forms began in 2002 when a research scientist at a laboratory in Long Island injected the contents of a syringe into a small white mouse. Within minutes, the mouse was dead, its body frozen by paralysis, the telltale sign of the lethal dose of poliovirus it had just received. The poliovirus's deceptively simple RNA-based replication cycle had gone into overdrive, hijacking the mouse's cells into making countless copies of itself, until the host cells, each swollen with ten thousand new viruses, ruptured, releasing them to infect more host cells. But what made this particular poliovirus attack so remarkable was that it had been induced using viral DNA built from scratch in the laboratory, the brainchild of a Stony Brook University virologist named Eckard Wimmer. The poliovirus genome had been deciphered in the summer of 1981, and Wimmer's team had only to download the genetic recipeâa simple string of about seventy-five hundred A's, G's, C's, and U'sâoff of the Internet.
The technology for synthesizing DNA had been perfected in the previous decades. Building such molecules, while not exactly child's play, was well within the repertoire of the modern molecular biologist by the turn of the millennium. Geneticist Craig Venter and his team started work on synthesizing an entire bacterial genome, a feat vastly more complex than Wimmer's simple virus. Even the smallest and simplest cells consist of hundreds of highly evolved enzymes, as well as the genetic code and all the other trappings used by modern organisms. Though it took twenty-four scientists ten years and forty million dollars, they succeeded, via a complex series of laboratory manipulations, in synthesizing their bacterial genome in 2010. It contained a staggering 1,077,947 base pairs.
*
To make their bacteria, Venter and his team began by adding the synthetic chromosomes they had built to a culture of natural
Mycoplasma
that was subjected to an electric shock. The shock allowed the artificial chromosome to enter the host cell. Then, as the host's cellular machinery went to work on the synthetic genome, daughter cells were produced containing only the artificial chromosome. This chromosome, having been endowed with all of the instructions for making all of the proteins needed to keep the cell running indefinitely, took over, and thus the first completely artificial organism came to life. They named it “Synthia.” It contained about eighty times as much genetic information as the poliovirus.
The implications for the future of biotechnology were truly remarkable. The potential applications reached into fields as diverse as synthetic fuel production and medicine. But many scientists were quick to point out that Synthia did little to answer the question of where the information required to build a cell came from in the first place. Venter, like Wimmer before him, essentially copied the blueprint that nature had provided them after four billion years of molecular tinkering. It was an astonishing feat of engineering but proved nothing about
how
life began.
S
ZOSTAK BEGAN CONTEMPLATING
a synthetically engineered cell as far back as the mid-1990s. What set his vision apart from that of men like Venter and Wimmer was that he wanted to understand the
origin
of life, not merely copy the blueprint provided by nature. The key question for him was how the blueprint would arise from scratch. He had some clues to draw upon, provided by a remarkable set of experiments carried out all the way back in the 1960s by Sol Spiegelman, the biochemist who had originally recruited Carl Woese to the University of Illinois.
Spiegelman and his colleagues performed a series of telling experiments that showed how RNA molecules might behave like organisms and evolve on their ownâin a test tubeâin a Darwinian fashion. Spiegelman started with a virus known as bacteriophage Qβâpronounced “cue beta”âwhich infects the common gut bacterium
Escherichia coli
. They purified Qβ's RNA genome and the protein that copies it, and then mixed these together in a test tube along with the precursors that the protein uses to construct a new Qβ RNA molecule. They let this mixture react for a little while and then transferred a few drops of the solution, now containing various imperfect copies of the original RNA molecule, to a new test tube that contained only the protein and precursors. They did this a total of seventy-four times, each time transferring a few drops from the last test tube to the next. In each exchange, a new population of mutant molecules was transferred, serving as the starting point for the “molecular evolution” that would take place in the next test tube.
The end of this process revealed something remarkable: the starting RNA molecules were about 4,500 nucleotides long, but the ones present in the seventy-fifth test tube were only 218 nucleotides long. There appeared to be a sort of competition in which shorter molecules tended to win. This made perfect sense, since short molecules could be copied faster, and thus could exponentially outcompete longer molecules. In essence, Spiegelman had created a form of natural selection among naked strands of RNA in his test tubes. His colleagues dubbed his creations “Spiegelman's monsters.”
In 1975, two scientists in chemist Manfred Eigen's lab performed a surprising experiment that built upon Spiegelman's work. This time they simply mixed the precursors and the protein without the master RNA template. Astonishingly, they found that over time, molecules very similar to Spiegelman's minimal RNA molecule appeared. This result showed that, given the right conditions, a meaningful information-containing molecule, like RNA,
can
spontaneously arise. The results obtained by Spiegelman and his colleagues became a touchstone for origin-of-life scientists in the RNA-world camp. The only problem was that the molecules were not truly self-replicating. They could make copies of themselves only in the presence of the copying protein, itself the complex product of a biological
blueprint. Thus, the concept of a
self
-copying RNA molecule became the holy grail of RNA-world research.
â