Life on a Young Planet (23 page)
Read Life on a Young Planet Online
Authors: Andrew H. Knoll
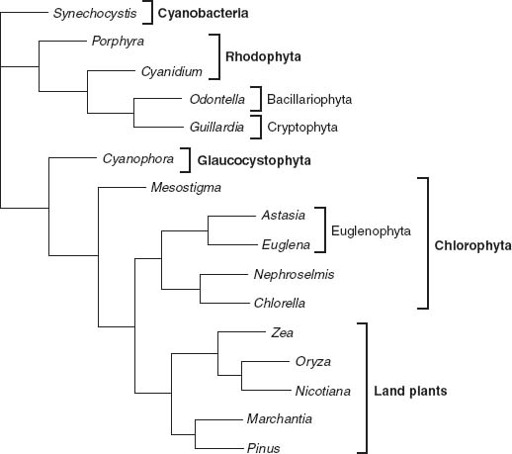
Figure 8.3.
Genealogical relationships among chloroplasts, based on molecular sequence comparisons. Note that the chloroplast tree does not show the same relationships as trees based on nuclear gene sequences. This strongly supports the idea that many eukaryotes acquired photosynthesis by engulfing other eukaryotic cells. Photosynthetic euglenids, for example, appear to be derived from endosymbiotic green algae, while the chloroplasts in cryptophyte and heterokont algae (here represented by the Bacillariophyta, or diatoms) seem to be descended from red algal symbionts. (Phylogenetic data courtesy of Paul Falkowski)
The cryptophytes are a small group of single-celled algae found in temperate and high-latitude waters. In a groundbreaking electron microscopic study, Sarah Gibbs of McGill University showed that cryptophyte chloroplasts are surrounded by four membranes, not just the two found in red and green algae. Moreover, a small dark body called the nucleomorph lies between the inner and outer membrane pairs. Surprisingly, Gibbs found that the nucleomorph contains DNA. These observations suggested to her that cryptophytes, like red and green algae, gained photosynthesis endosymbiotically. But in the case of cryptophytes, she hypothesized, the symbiont was itself a eukaryotic alga, not a cyanobacterium. Gibbs reasoned as follows: the two innermost membranes surrounding cryptophyte chloroplasts represent the two chloroplast membranes of the engulfed algal symbiont; the nucleomorph and associated material between membrane pairs are remnants of the symbiont’s nucleus and cytoplasm; and the two outer membranes reflect the symbiont’s cell membrane and the enveloping membrane synthesized by its captor. Gene sequence comparisons confirm Gibbs’s hypothesis: the chloroplast genes of cryptophyte algae cluster with those of cyanobacteria, genes retained in the nucleomorph branch with nuclear genes of red algae, and genes of the cryptophyte nucleus represent a third distinct eukaryotic lineage. Cryptophytes did indeed acquire photosynthesis by swallowing a eukaryotic alga.
Figure 8.4 summarizes the spread of photosynthesis through the Eucarya. The chloroplasts of red algae have photosynthetic pigments similar to those of cyanobacteria and, as already noted, are surrounded by two membranes; they reflect the
primary
endosymbiotic incorporation of a cyanobacterium by a eukaryotic cell. Green algal chloroplasts have somewhat different pigments—chlorophyll b was added and proteinaceous pigments lost—but their double membrane also reflects primary endosymbiosis. Given increasing evidence that red and green algae are closely related, a single symbiotic event may account for both groups.
Only two groups of algae are known to contain nucleomorphs. Nonetheless, biologists agree that algae other than reds and greens acquired photosynthesis via
secondary
endosymbioses that incorporated eukaryotic symbionts, a conclusion supported by sequence comparisons of chloroplast genes and studies of membrane organization. There is even one case of
tertiary
endosymbiosis, found within the dinoflagellates,
common members of marine plankton (and the photosynthetic symbionts of corals). These biological Matryoshka dolls originated when a flagellated protist engulfed a so-called haptophyte alga—which itself arose when a protozoan swallowed a unicellular alga closely related to living reds—which, in turn, evolved via the endosymbiotic incorporation of a cyanobacterium into a eukaryotic host! Lewis Thomas once wrote, “I take it as an article of faith that [the committee] is the most fundamental aspect of nature that we know about.” Dinoflagellates epitomize this view of life.
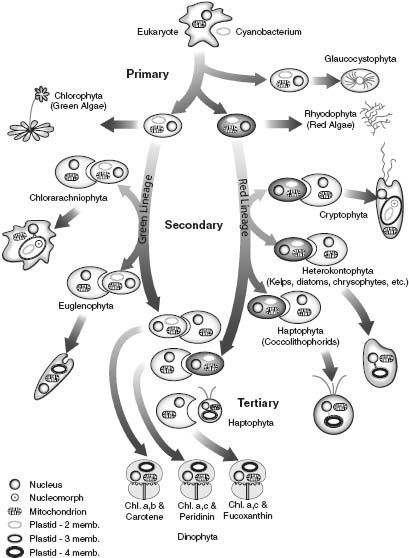
Figure 8.4.
A summary of the endosymbiotic events by which photosynthesis spread through the Eucarya. (Reproduced with permission from an illustration by Charles Delwiche)
How surprised should we be by the spread of photosynthesis through the eukaryotic domain? On first encounter, it seems almost implausibly odd—an improbable sequence of events completed in some distant, more permissive ocean. But, as noted earlier, the symbiotic acquisition of photosynthesis has occurred repeatedly through time. The lichens found on hillside rocks comprise symbiotic associations between fungi and green algae or cyanobacteria. Some of these associations are casual, and the two partners can be grown separately; others have become so intimate that segregation of host and symbiont is no longer possible.
Like reef corals,
Tridacna
, the giant clam of tropical oceans, farms microscopic algae within its tissues. Algal symbionts occur as well in flatworms, sponges, and untold protozoans (including ciliates, radiolarians, and foraminifers). Even some sea squirts, distant relatives of our own, harbor symbiotic cyanobacteria. If you want to see something
truly
unusual, look in the mirror—vertebrate animals don’t seem to be capable of forming symbioses with photosynthetic microorganisms.
As Lynn Margulis recognized, the story of mitochondria parallels that of chloroplasts. Just as photosynthesis in eukaryotic cells is localized within chloroplasts, aerobic respiration—the metabolism that fuels our own bodies—is confined to mitochondria. In structure and biochemistry, these small organelles closely resemble members of a bacterial clade called the proteobacteria. Like chloroplasts, mitochondria are bounded by two membranes, and they also contain DNA, RNA, and ribosomes. As early as 1925, Ivan Wallin, a professor of anatomy at the University of Colorado, proposed that mitochondria are fundamentally bacterial. He even claimed that he could culture them as free-living organisms. No one else could do this (in fact, it can’t be done), and Wallin,
like Merezhkovsky before him, faded into obscurity. But once again, molecular biology showed that Wallin was not wrong (at least not in his general thesis); he was just ahead of his time. Molecular phylogeny shows clearly that mitochondria originated as bacterial cells that evolved through time from casual symbionts to obligatory organelles. In this case, the encompassing host supplied the sugar; the protomitochondrial symbionts returned energy (in the form of ATP) in copious supply.
In
chapter 2
, we compared the dazzling metabolic diversity of prokaryotic organisms with the limited capabilities of eukaryotes. Nucleated organisms may photosynthesize, respire aerobically, or, occasionally, ferment, but that’s about the extent of their abilities. Our modern understanding of mitochondria and chloroplasts makes this comparison seem even more lopsided, as the two metabolisms that power most eukaryotic cells can be traced to lateral transfer on a grand scale—the wholesale importation of bacterial cells. It reminds me of a scene in
A Streetcar Named Desire
, in which Blanche DuBois declares that she has “always benefited from the kindness of strangers.” Eukaryotes are the Blanche DuBois of biology.
If chloroplasts and mitochondria originated as bacterial symbionts, how did the rest of the eukaryotic cell take shape? Recall from
chapter 2
that eukaryotes share a number of fundamental characters not found in archaeans or bacteria. Their defining feature, of course, is the nucleus: the membrane-bounded compartment that contains the cell’s genes. Inside the nucleus, long strands of DNA are wound tightly around tiny proteinaceous beads to form linear chromosomes. The electron microscope reveals an intracellular landscape that further differentiates eukaryotes from other organisms (
figure 8.1
). Nucleated cells have a distinctive arrangement of proteinaceous strands called microtubules in their flagella, their cell functions are localized within well-defined organelles such as the Golgi apparatus (flattened sacs involved in intracellular transport and secretion), and, as we’ve already seen, they have mitochondria and chloroplasts. In detail, eukaryotes are also biochemically unique: transcription, translation, and ribosome structure are all distinct from comparable features in prokaryotes.
Perhaps the most important differences between eukaryotes and other
cells concern the way in which the cell’s contents are stabilized. Archaeans and bacteria enclose their cytoplasm in a rigid wall. In contrast, eukaryotes evolved an internal scaffolding called the cytoskeleton, and that, as Robert Frost once wrote, has made all the difference. Built from tiny filaments of actin and other proteins, the cytoskeleton is a remarkably dynamic structure, continually able to form and re-form in ways that change the cell’s shape. Many of us remember film clips of amoebas, viewed on slow days in high school biology class. The graceful undulations of the amoeba, its pseudopodia extending to capture prey, reflect the coordinated action of a dynamic cytoskeleton and a flexible membrane system. This coordination is key to the evolutionary success of eukaryotes precisely because it enabled these cells to engulf particles—paving the way for the acquisition of mitochondria and chloroplasts.
Endosymbiosis may explain the biology of mitochondria and chloroplasts, but it does little to illuminate the many other attributes of eukaryotic cells. Indeed, in terms of classical endosymbiotic theory, the cell that swallowed the protomitochondrion must already have been a card-carrying eukaryote with respect to other key features. This view of eukaryotic evolution makes an explicit genealogical prediction. Eukaryotes that contain chloroplasts and mitochondria should be distal branches on a limb that also includes cells with mitochondria alone. (No chloroplast-bearing cells lack mitochondria.) And the earliest branches of the eukaryotic tree should contain nucleated cells without mitochondria—the prototypical eukaryotes that set all the endosymbioses in motion.
During the 1980s, Mitchell Sogin of the Marine Biological Laboratories in Woods Hole, Massachusetts, pioneered molecular studies of eukaryotic phylogeny. Inspired by Carl Woese, Sogin turned to sequence comparisons of ribosomal RNA genes to sort out eukaryotic relationships. Remarkably, the trees he constructed fit the genealogical predictions of the endosymbiotic hypothesis beautifully: algae reside on upper branches, middle branches include amoebas and other cells with mitochondria but no chloroplasts,
1
and most intriguing, the lowermost
branches contain nucleated cells with neither mitochondria nor chloroplasts.
Giardia lamblia
, an intestinal parasite well known to backpackers who drink untreated water, serves to illustrate these early branching eukaryotes.
Giardia
is a tiny bullet-shaped cell that thrives in the oxygen-poor environments provided by vertebrate digestive tracts. It has a relatively simple internal organization—for example, there is only limited evidence of a Golgi apparatus—and it retains details of DNA transcription otherwise known only from prokaryotic organisms. In many ways, these cells provide an excellent model for an early eukaryotic cell. But there is a problem. While it is attractive to interpret
Giardia’
s simple biology in terms of ancient origins, we might instead view its peculiar features as adaptations for a parasitic mode of life. Because they obtain many of their physiological needs from their hosts, parasites commonly display a stripped-down biology. Perhaps, then,
Giardia
once had mitochondria but lost them. As it turns out,
Giardia
has some free-living relatives that share many of its unusual traits, so parasitism doesn’t explain everything. Nonetheless, because
Giardia
’s free-living cousins also live in oxygen-depleted habitats, the question persists: are eukaryotes that lack mitochondria primitively simple, or were their mitochondria jettisoned as unnecessary baggage in the anoxic environments where these cells thrive?
This question might seem intractable, but in fact there is an elegant means of addressing it. Recall that as endosymbionts evolved into organelles, some of their genes were transferred to the nuclear genome. This being the case, we can ask about lost mitochondria in a relatively straightforward way: do the nuclei of mitochondria-free eukaryotes contain genes transferred from since-departed endosymbionts?
The answer, in some cases at least, is yes. Genetic vestiges of lost mitochondria were first discovered in
Entamoeba histolytica
, an anaerobic parasite nested among conventional amoebas in the eukaryotic tree. Specifically, the nuclear genome of
E. histolytica
contains a gene for a chaperone protein called cpn60 that is closely related to the cpn60 genes of mitochondria and free-living proteobacteria. The simplest explanation for this observation is that entamoebas had mitochondria and lost them—but they didn’t lose all their mitochondrial
genes
.