Sun in a Bottle (2 page)
Authors: Charles Seife
Uranium—in particular, one variety known as uranium-235—is an ideal material for a weapon. Its atoms are very sensitive; hit one with a subatomic particle and it fissions into fragments. Unlike decaying radium, which tends to cleave cleanly into two parts, a fissioning uranium atom shivers into a number of smaller chunks, including a handful of neutral particles known as neutrons. These neutrons then fly away from the shattered atom.
In a vacuum, the neutrons continue merrily on their way without bumping into anything else. However, a chunk of uranium is not a vacuum; it is a space crowded full of billions and billions of other uranium atoms. Once a single atom splits apart, within a tiny fraction of a second the resulting neutrons might slam into two or three other uranium atoms. These collisions cause those atoms to split, and in the process, each releases two or three more neutrons. All these neutrons slam into other atoms, splitting them, releasing even more neutrons. If the conditions are right—if enough uranium atoms are in a small enough space—then the process snowballs out of control in less than a blink of an eye. One atom fissions, and its neutrons cause two more to split. These cause four more to fission, causing eight to break apart, then sixteen, thirty-two, sixty-four, and so forth. After ten rounds, over two thousand atoms have split, releasing neutrons and energy. After twenty rounds, it’s more than two million atoms; after thirty rounds, two billion; after forty, more than a trillion. This is a chain reaction.
A chain reaction, if it gets big enough, can level a city. Every time a uranium nucleus splits, it releases energy. Like radium, a uranium atom loses mass when it splits. In a tiny instant, the mass is converted into energy, just as
E
=
mc
2
predicts. The more atoms that split in the chain reaction, the more energy is released. After forty rounds of splitting uranium atoms, the energy is roughly enough to light an incandescent lightbulb for about a second. After eighty rounds, a mere fraction of a second after the chain reaction begins, the result is more energetic than the explosion of ten thousand tons of TNT, roughly the size of the blast that eventually destroyed Hiroshima.
E
=
mc
2
predicts. The more atoms that split in the chain reaction, the more energy is released. After forty rounds of splitting uranium atoms, the energy is roughly enough to light an incandescent lightbulb for about a second. After eighty rounds, a mere fraction of a second after the chain reaction begins, the result is more energetic than the explosion of ten thousand tons of TNT, roughly the size of the blast that eventually destroyed Hiroshima.
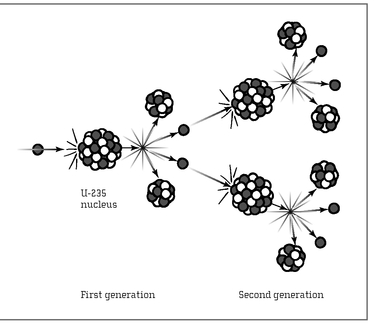
FISSION CHAIN REACTION:
When a neutron strikes a U-235 nucleus, the nucleus splits, releasing more neutrons, which strike more nuclei, and so on.
When a neutron strikes a U-235 nucleus, the nucleus splits, releasing more neutrons, which strike more nuclei, and so on.
In 1939, though, the idea of fission—and a chain reaction that would release a tremendous amount of energy—was just a theory. Before World War II began, scientists were uncertain whether the theory was right—and if so, how to turn that theory into the hard reality of a useful weapon. It took two years of cogitation and experimentation for the consensus to build: it was possible to build a powerful bomb out of uranium-235 or plutonium-239 (an atom created in the lab by bombarding uranium with neutrons). Nuclear theory progressed quite rapidly; by 1942, the physicist Enrico Fermi was busy building the first nuclear reactor in a squash court
1
at the University of Chicago. Fermi’s project was a major step toward releasing the power of the atom—and eventually bringing the wrath of the sun upon the Earth.
1
at the University of Chicago. Fermi’s project was a major step toward releasing the power of the atom—and eventually bringing the wrath of the sun upon the Earth.
The core of a nuclear reactor is little more than a controlled chain reaction: a pile of fissioning material that is not quite at the stage of entering a runaway explosion. Scientists arrange the pile so that the number of neutrons produced by splitting atoms is almost precisely the right amount to keep the reaction going without getting faster and faster; each generation of fission has roughly the same number of atoms fissioning as the last. In physics terms, the pile is kept right near
critical
condition. Scientists can manipulate the rate of the reaction by inserting or removing materials that absorb, reflect, or slow neutrons. Pull out a rod of neutron-absorbing material and more neutrons are available to split atoms and release more neutrons: the pile goes critical. Drop the rod back in and more neutrons are absorbed than released: the reaction sputters to a halt.
critical
condition. Scientists can manipulate the rate of the reaction by inserting or removing materials that absorb, reflect, or slow neutrons. Pull out a rod of neutron-absorbing material and more neutrons are available to split atoms and release more neutrons: the pile goes critical. Drop the rod back in and more neutrons are absorbed than released: the reaction sputters to a halt.
At 3:36 PM on December 2, 1942, Fermi and his colleagues pulled a neutron-absorbing rod out of a pile of graphite and uranium oxide. The radiation counters chattered. Fermi had created the first self-sustained nuclear reaction. The pile had gone beyond critical; more neutrons were being produced by each generation of fission than the last. The reactor was producing more and more and more energy. About a half hour later, Fermi ordered the control rods back into the pile, and the reaction stopped. At its peak the reactor was producing about half a watt of power, almost enough to light a dim Christmas-tree lightbulb. Nevertheless, the possibilities were enormous: Fermi’s reactor showed that nuclear power could, in theory, light up a city. Or destroy it.
It was for the latter purpose that the Manhattan Project was born. At its head was a quirky and difficult scientist, J. Robert Oppenheimer, a man who would achieve fame through fission and be destroyed by fusion.
Oppenheimer was not an obvious choice to lead America’s race to build an atom bomb. He was a good physicist, but he was a theorist—and the Manhattan Project was, fundamentally, an engineering project. Oppenheimer was about as far from the stereotypical get-your-hands-dirty engineer as possible.
The aristocratic Oppenheimer grew up in a wealthy family, but what was particularly striking about him was his quick mind. He mastered more than half a dozen languages, including Sanskrit. He was an adept theoretician but struggled with the more practical side of science; he had difficulty even with basic tasks such as soldering copper wires. After graduating from Harvard, he went to Cambridge in England to work in the lab of the famous experimentalist J. J. Thomson. There, the already high-strung Oppenheimer became unglued.
Oppenheimer had a difficult time at Cambridge; in his mind, his experiments were failures, and he contemplated suicide. He also contemplated murder. In 1925, he suddenly tried to strangle a childhood friend, and his behavior got even more bizarre from there. On a vacation in Corsica with two friends, he abruptly announced, “I’ve done a terrible thing.” He said that he had poisoned an apple and put it on the desk of another brilliant physicist at Cambridge, Patrick Blackett. When everyone got back to the university, they found out that Blackett was unharmed, and Oppenheimer’s friends were left wondering whether the apple was real or just a figment of Oppenheimer’s feverish imagination.
The bizarre behavior became less acute once Oppenheimer relocated to the University of Göttingen in Germany. In the 1920s, Germany was the world leader in theoretical physics—home to Einstein, Max Planck, Werner Heisenberg, Max Born, and many of the other leading lights of the day—and Oppenheimer established himself as a brilliant young physicist. However, he was still depressive. He was also vain, arrogant, and occasionally nasty. He had a habit of making people feel small and insignificant; he detested his “beastliness” but was unable to control it. Nevertheless, soon after moving back to the United States to become a professor at the University of California at Berkeley, he acquired a circle of devotees thanks to his brilliance and wit.
Despite Oppenheimer’s prickliness, everyone—even the occasional general—was impressed with the young professor. “He’s a genius,” wrote General Leslie Groves, the military head of the Manhattan Project and the man who chose Oppenheimer to lead the scientific effort. “Why, Oppenheimer knows about everything. He can talk to you about anything you bring up. Well, not exactly. I guess there are a few things he doesn’t know about. He doesn’t know anything about sports.” This was by no means the most serious of his flaws, as far as the military was concerned.
Oppenheimer was a security risk—he was absolutely surrounded by Communists. His brother and sister-in-law were members of the Communist Party. His first fiancée, Jean Tatlock, had been a member, too. His wife Kitty’s first husband had been an official in the party and had been killed fighting on the leftist side during the Spanish Civil War. The army knew about all these connections, yet Groves insisted that Oppenheimer lead the most sensitive military project of World War II. In October 1942, Oppenheimer accepted his new post and began assembling the biggest scientific project in the history of mankind.
Laboratories devoted to the atom bomb effort sprang up around the country. Los Alamos, perched on a mesa in the New Mexico desert, was the intellectual heart of the Manhattan Project. Other facilities, such as one at Oak Ridge in Tennessee and another at Hanford in Washington, were crucial to figuring out the best way to separate bombworthy uranium-235 from the much more common uranium-238 and how to manufacture plutonium-239.
2
However, the big minds roamed at Los Alamos: Oppenheimer, Hans Bethe, Richard Feynman, Stanislaw Ulam, John von Neumann, Enrico Fermi, and Edward Teller.
2
However, the big minds roamed at Los Alamos: Oppenheimer, Hans Bethe, Richard Feynman, Stanislaw Ulam, John von Neumann, Enrico Fermi, and Edward Teller.
Teller, a Hungarian émigré and, arguably, a better theoretician than Oppenheimer, was brought to the University of Chicago in mid-1942 by the Manhattan Project just as it was getting under way. When Teller arrived, nobody assigned him a task, so he set to work trying to design the ultimate weapon, more powerful even than the one the project’s scientists were trying to build. He envisioned a superbomb that used fusion instead of fission. If it worked, it would dwarf an atom bomb just as surely as an atom bomb would dwarf conventional explosives. Teller became obsessed with wielding the power of the sun. It was an obsession that molded him into one of the darkest and most twisted figures of American science. “He’s a danger to all that’s important,” said his fellow physicist Hans Bethe. “I really do feel it would have been a better world without Teller.”
Teller was born in Budapest, the child of a successful lawyer. In 1919, when he was eleven years old, the Communist Béla Kun swept to power and declared Hungary a Soviet state. “The communists overturned every aspect of society and the economy,” Teller later wrote. “My father could no longer practice law.” Two soldiers moved into the Tellers’ home, and young Edward came to know hunger. “There was no food (or any other kind of goods) for sale in the stores now owned by the communists. . . . As I recall, cabbage was often all we could find. I still dislike cabbage.”
After rampant inflation, a coup attempt, a purge, and a military defeat, Kun’s regime ended before the year was out. But the whole experience left Teller with an almost monomaniacal hatred of Communism. In large part, his actions over the next few decades—his attempt to build an arsenal of unlimited power—would be driven by that hatred.
3
3
Thus Teller’s vision of a superweapon was possible because there is more than one way to extract energy from the atom. Fission is the easy way. Just get enough fissile material (such as uranium-235 or plutonium- 239) in a small enough space and a chain reaction will start on its own. Heavy atoms will split into fragments, converting mass into energy and creating an enormous explosion. The main problem is getting that fissile material. Neither uranium-235 nor plutonium-239 was easy to obtain, especially with the state of knowledge in 1942 and 1943.
Fusion is another way to convert mass into energy; it’s the opposite of fission. In fission, heavy atoms split and the sum of the parts is lighter than the original atoms. In fusion, light atoms stick together, and the whole resulting atom is lighter than the sum of the parts that made it. The missing matter—the stuff that disappears when the light atoms combine—becomes energy.
Fusion is several times more powerful than fission; more of the mass of each reacting atom is converted into energy. Better yet, it is much easier to find the fuel for fusion—light atoms like hydrogen—than it is to find the uranium or plutonium fuel for fission. The oceans are filled with hydrogen’s heavier sibling, deuterium, a great fuel for fusion reactions. It’s not terribly difficult to extract a practically unlimited amount of the stuff.
Of course, there is a downside. The fusion reaction is extremely difficult to start, and even harder to keep going long enough to produce large quantities of energy. Atoms tend to repel each other, so it is very hard to get them close enough so that they stick together. You need an enormous amount of energy to slam two atoms together forcefully enough to overcome that repulsion and get them to fuse.
For a fission reaction, you just need to get a lump of uranium big enough. For fusion, you need to manipulate your fuel in some tricky ways. First, you’ve got to compress the fuel into a tiny parcel. This keeps the atoms in close proximity to one another (so they have a chance of colliding). That, in itself, is not so hard; the trick is to keep the atoms very hot as well. Only at tens or hundreds of millions of degrees are the atoms moving fast enough to have a chance of fusing when they do collide. When you heat something, it expands—the atoms try to escape in all directions. Thus, it is very hard to keep a very hot thing compressed very tightly. So, the basic problem in fusion is that it is very difficult to heat something to the right temperature and, simultaneously, keep the atoms close enough together. Without both things working concurrently, a fusion reaction won’t get going.
Other books
The City of Lost Secrets: A Mara Beltane Mystery by Katie McVay
Red, White and Beautiful by Botefuhr, Bec
Lie to Me (an OddRocket title) by Brahm, Suzanne
Curtains For Three by Stout, Rex
All Because of You (Lakeview #2) by Hill, Melissa
Daniel Klein by Blue Suede Clues: A Murder Mystery Featuring Elvis Presley
Carolina Heat by Christi Barth
OUR LAST NIGHT: an edge of your seat ghost story thriller by ADAMS, TAYLOR
Oda a un banquero by Lindsey Davis
To Love a Scoundrel (Zebra Historical Romance) by Kristina Cook