The Flamingo’s Smile (6 page)
Read The Flamingo’s Smile Online
Authors: Stephen Jay Gould
Our world is not an optimal place, fine tuned by omnipotent forces of selection. It is a quirky mass of imperfections, working well enough (often admirably); a jury-rigged set of adaptations built of curious parts made available by past histories in different contexts. Darwin, who was a keen student of history, not just a devotee of selection, understood this principle as the primary proof of evolution itself. A world optimally adapted to current environments is a world without history, and a world without history might have been created as we find it. History matters; it confounds perfection and proves that current life transformed its own past. In his famous disquisition on the ages of man—“All the world’s a stage”—Jaques, in
As You Like It
, speaks of “this strange eventful history.” Respect the past and inform the present.
Postscript
In the light of my ever-growing doubts about the existence of sexual cannibalism (despite its plausibility in theory)—as prominently displayed in the personal odyssey of the essay itself—I was delighted by a report from the 1984 annual meeting of the Society for Neuroscience. E. Liske of West Germany and W.J. Davis of the University of California at Santa Cruz videotaped and analyzed courtship behavior for dozens of matings in Chinese praying mantises. No female ever decapitated or ate a male. Instead, frame-by-frame analysis revealed a complex series of behaviors, seemingly directed (at least in part) towards suppressing the natural rapacity of females. Male behavior includes visual fixation, antennal oscillation, slow approach, repetitive flexing of the abdomen, and a final flying leap towards the female’s back. Liske and Davis suggest that previous reports of decapitation may represent aberrant behavior of captive specimens (though sexual cannibalism may still be normal behavior in strains or species other than those studied by Liske and Davis. There is no such thing as
the
praying mantis, given nature’s propensity for diversity.). In any case, I am even more persuaded that sexual cannibalism is a phenomenon without proven examples, and that the reasons for its rarity (or non-existence) form a far more interesting subject (and an appropriate shift of emphasis) than the one that first inspired my research for the essay—reasons for the presumed (and now dubious) existence itself.
I often argue that the best test for legends is the extent of their seepage into popular culture. In
Sherlock Holmes and the Spider Woman
(1944), one of the innumerable, yet wonderful, Rathbone-Bruce anachronisms that pit Holmes against Hitler and assorted enemies, Holmes unmasks an entomologist
poseur
(and murderer of the true scientist) by catching several subtle fallacies in his speech. The phony calls terraria “glass cages,” but then really gives himself away when he says of black widow spiders: “They eat their mates, I’m told.” Holmes responds: “You said you were told the black widows eat their mates. Any scientist would know it.” I shall be waiting for the next update (who is playing Charlie Chan these days?).
AS AN FIGHT-YEAR-OLD COLLECTOR
of shells at Rockaway Beach, I took a functional but non-Linnaean approach to taxonomy, dividing my booty into “regular,” “unusual,” and “extraordinary.” My favorite was the common slipper limpet, although it resided in the realm of the regular by virtue of its ubiquity. I loved its range of shapes and colors, and the pocket underneath that served as a protective home for the animal. My appeal turned to fascination a few years later, when I both entered puberty and studied some Linnaean taxonomy at the same time. I learned its proper name,
Crepidula fornicata
—a sure spur to curiosity. Since Linnaeus himself had christened this particular species, I marveled at the unbridled libido of taxonomy’s father.
When I learned about the habits of
C. fornicata
, I felt confident that I had found the key to its curious name. For the slipper limpet forms stacks, smaller piled atop larger, often reaching a dozen shells or more. The smaller animals on top are invariably male, the larger supporters underneath always female. And lest you suspect that the topmost males might be restricted to a life of obligate homosexuality by virtue of their separation from the first large female, fear not. The male’s penis is longer by far than its entire body and can easily slip around a few males to reach the females.
Crepidula fornicata
indeed; a sexy congeries.
Then, to complete the disappointing story, I discovered that the name had nothing to do with sex. Linnaeus had described the species from single specimens in museum drawers; he knew nothing of their peculiar stacking behavior.
Fornix
means “arch” in Latin, and Linnaeus chose his name to recognize the shell’s smoothly domed shape.
*
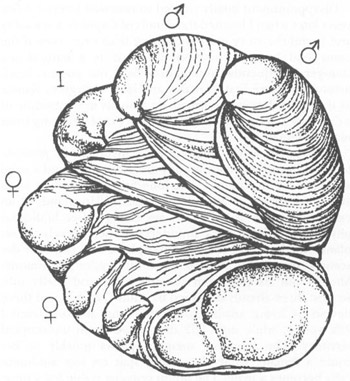
A
Crepidula
stack, with sexes identified. Bottom members are female; the smaller individuals on top are male. The animal in the middle (labeled I) is undergoing a transition from male to female.
REPRINTED FROM NATURAL HISTORY
.
Disappointment finally yielded to renewed interest a few years later when I learned the details of
Crepidula
’s sexuality and found the story more intriguing than ever, even if the name had been a come-on.
Crepidula
is a natural sex changer, a sequential hermaphrodite in our jargon. Small juveniles mature first as males and later change to female as they grow larger. Intermediate animals in the middle of a
Crepidula
stack are usually in the process of changing from male to female.
The system works neatly for all involved.
C. fornicata
tends to live in relatively muddy areas but must find a solid substrate for attachment. The founding member of a stack affixes to a rock or an old shell. Elaine Hoagland, in an exhaustive study of
Crepidula
’s sex changes (see bibliography), observed that these founders can then actively attract planktonic larvae as they metamorphose and begin to descend—presumably by some chemical lure, or pheromone. She set out six pots with suitable rocky and shelly substrates: three already occupied by adult
Crepidula
and three devoid of living snails. Pots containing adults attracted 722 young, while only 232 descended upon unoccupied territory. The founding member grows quickly to become a female, while the young spat on top automatically becomes a male. The union remains stable for a time, but eventually the male grows up and turns into a female. The pair of females can then attract other small
Crepidulas
, which become well-supplied males. The stack grows, always maintaining an ample number and ratio of males and females.
This curious system provides a particularly interesting example of a general phenomenon in nature. Sex change might go either way (or both) during growth, from male to female or from female to male. Both phenomena occur, but
Crepidula
’s pattern of male first and female later, called
protandry
(or male first) is by far the more common. (Creatures that are first female and then male are
protogynous
, or female first.) Protandry seems to represent the prevalent path of changing sex, with protogyny as a rarer phenomenon evolved under special (but not particularly uncommon) circumstances. Why should this be?
The answer pricks one of our old prejudices and false extrapolations to all of nature from the animals we know best, ourselves and other mammals. We think of males as large and powerful, females as smaller and weaker, but the opposite pattern prevails throughout nature—males are generally smaller than females, and for good reason, humans and most other mammals notwithstanding. Sperm is small and cheap, easily manufactured in large quantities by little creatures. A sperm cell is little more than a nucleus of naked DNA with a delivery system. Eggs, on the other hand, must be larger, for they provide the cytoplasm (all the rest of the cell) with mitochondria (or energy factories), chloroplasts (for photosynthesizers), and all other parts that a zygote needs to begin the process of embryonic growth. In addition, eggs generally supply the initial nutriment, or food for the developing embryo. Finally, females usually perform the tasks of primary care, either retaining the eggs within their bodies for a time or guarding them after they are laid. For all these reasons, females are larger than males in most species of animals.
This system can be overridden when males evolve a form of competition with other males that favors large size for success in gaining sexual contact with females. Such forms of competition are wasteful in terms of such theoretical concepts as “the good of the species.” But Darwinism is about the struggle of individual organisms to pass more of their genes to future generations. The best indication that our world is Darwinian lies with these cases of evolution for individual advantage alone—as when males become larger because they compete as individuals in battle or sexual display for access to females.
Competition of this form generally requires a fair degree of intelligence, since such complex actions imply flexible and extensive behavioral repertoires. Thus, we tend to find the unusual or reversed pattern of larger males in so-called higher creatures of substantial brain. This correlation of complexity and mental power probably explains why, of all groups with a large number of sequential hermaphrodites, only vertebrates have evolved protogyny as a more common pattern than protandry. When we look at the natural history of most protogynous fish, we see that behavioral imperatives based on male-male competition have conditioned the pattern of females first, changing to larger males. Douglas Y. Shapiro, for example, studied sex reversal in
Anthias squamipinnis
, a shallow-water tropical marine fish that lives among coral reefs in stable social groups averaging eight adult females to one male (see bibliography). Males may compete intensely to retain and maintain their groups. The removal of a male induces a female to change sex, and this transition includes a set of features all conducive to keeping charge of several females: change to more gaudy color, longer fin spines, more elaborate caudal fin streamers, and larger size.
The distribution of protandry and protogyny provides an even better illustration of nature’s preference for larger females than the simple documentation of permanently smaller males in insects or angler fishes. Permanent males and females represent static systems that may maintain their relationships of size for a set of other reasons. But when we find that
active
change of sex usually proceeds from male to female, we must seek some direct reason rooted in the general advantages of larger female size.
We might seek a still better illustration, one, unfortunately, that animals, as a constraint of their mode of growth, will not be able to provide. Ideally, we would like to find a creature that changes sex in either direction but becomes female when it grows larger and male when it becomes smaller. Can we hope for such an ideal case in nature, a total confirmation of a general principle all wrapped up in a single creature? (As long as we must wrap the principle in several creatures, we shall be haunted by the distressing possibility that we have it all wrong—that protogyny dominates in fishes, not because they are advanced behaviorally and illustrate Darwin’s principle of individual competition, but as a consequence of some unknown and peculiar property of fishness. If, however, we can find both phenomena in the same creature, a unified explanation seems assured.) But do we have a right to expect such an ideal example from nature? Animals, after all, with very rare exceptions, never grow smaller and will therefore not serve. One of the earliest articles on sex change in
Crepidula
, written in 1935, ended with these words: “Sexual transformation in
Crepidula
, like metamorphosis in other animals, can be hastened or retarded experimentally, but it cannot be reversed.”
Nature has come through again—she always does. The ideal organism has surfaced. It is a plant, the general subject, unfortunately, of my woeful and abysmal ignorance. Plants can undergo substantial reduction in size, for several reasons and without expiring. Our example is a common and attractive inhabitant of local eastern woodlands,
Arisaema triphyllum
, the jack-in-the-pulpit. Results were recently reported by my friend David Policansky in the staid Proceedings of the National Academy of Sciences (see bibliography). (I confess that my previous attention to this plant was virtually confined to wondering whether its plural form included one jack and several pulpits, as in most words, or several jacks and one pulpit, as in those old bugbears of high school grammar, attorneys-general and mothers-in-law. I note that this matter must confuse others as well, because the two references I have found to Policansky’s work both studiously avoid the issue and, in defiance of the rules of grammar, use the singular in all cases. I will opt for several pulpits, even though I know that each one carries a jack. Or are they like sheep after all?)
*
The flowers of most (but by no means all) plants contain both male and female structures. But jack-in-the-pulpits are either one or the other. The sexual part of the blossom contains either anthers, the male’s sexual structure, or ovaries capped with stigmas. Smaller plants, the males, have one leaf, while the larger females usually grow two leaves. During a three-year study at Estabrook Woods in Concord, Massachusetts, Policansky marked and recorded 2,038 jack-in-the-pulpits; 1,224 were male with a mean height of 336 mm, while 814 females averaged 411 mm in height.
The so-called “size advantage” model of sex change predicts that, for the usual case of smaller males, a transition from male to female should occur where any further increase in size begins to benefit a female (in terms of seeds that can be produced) more than a male. (Remember that small males can produce a superabundance of sperm, and larger size therefore offers relatively little additional advantage, while the benefit to females can be substantial.) Citing data on the increase in sperm and seed number with size, Policansky calculated that, in theory, this transition in jack-in-the-pulpits should occur at a height of 398 mm. He then found that, in nature (or, at least, in Concord), 380 mm is the watershed—a very close agreement with theory. Below this height, he found more males than females; above, more females than males.
He was also able to ascertain directly that male plants tended to change to female as they grew larger in the normal course of life. Moreover, and this is the key observation, individuals changed from female to male in the more unusual circumstances that occasionally lead a plant to become smaller. Size decrease occurred for three reasons: when part of the plant was eaten (if Jill breaks her crown, Jack comes after); when the plant became shaded and, consequently, stunted in growth; and when it had set an unusually large number of seeds the season before, thus also inhibiting growth in size by diverting most energy to the seeds themselves.
Thus, with change in both directions conforming to the size advantage model and following nature’s usual pattern of smaller males and larger females, the jack-in-the-pulpit provides, all by itself, a lovely illustration of the errors in our usual, narrow perceptions and assumptions about the relative size of sexes—and an excellent confirmation of an important principle in Darwinian biology. It will also help us to understand why, if
man
is truly the measure of all things, Jill will need an enlarged pulpit.