Read When All Hell Breaks Loose Online
Authors: Cody Lundin
When All Hell Breaks Loose (67 page)
Lately there are some very cool LED (light-emitting diode) lights on the market that spit out a surprising amount of light for their size and have a tremendous bulb and battery life. Some flashlights that use standard bulbs can be modified to use this technology. The mini-mag brand of flashlight, in the AA battery size, has an LED addition that can be purchased from many outdoor stores. This option replaces the standard bulb with three LED bulbs that are renowned for their long life. You can also purchase an end-cap piece that allows you to push a button to turn on the flashlight instead of twisting the bulb end of the light. Gone will be the option of a focused or wide beam from the standard mini-mag bulb, and if you purchase the push-button end cap, the neat little metal loop for easily attaching a lanyard will also disappear.
The Beautiful yet Baffling Battery: Choosing, Storing,
and Rotating Your Batteries
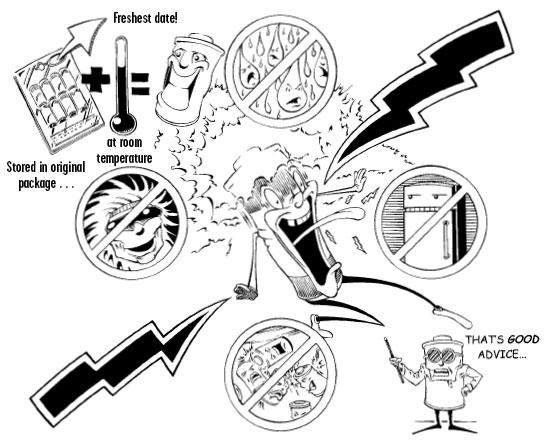
There are many types of batteries on the market although not all of the batteries listed below are used in flashlights; some are for hearing aids, pacemakers, and the like. While most are categorized as "primary cells," meaning they are one-use wonders and can't be recharged, there are many options for "secondary cells" or batteries that can be recharged. Types of batteries under the primary-cell heading include alkaline/manganese (the most common household battery type), carbon-zinc, lithium, mercuric-oxide, silver-oxide, zinc-air, and other types of button batteries. Secondary-cell choices include nickel-cadmium and small sealed lead-acid, along with a few alkaline varieties. Many of these batteries, when spent or having grown tired of several recharges, are extremely toxic. Nickel-cadmium, for example, a popular but outdated rechargeable battery (it's been replaced by nickel-metal hydride), is supposed to be disposed of at a household hazardous waste collection site. Rechargeable batteries are not on the top of our list, as much as I love them (as mentioned, my entire home runs on solar power so this book is being written with sunlight and, of course, the batteries that hold the solar power when I write at night!). Remember, the reason you are using a light is because the grid has gone down; where is the power going to come from to recharge those rechargeables? Also, nickel-cadmium and nickel-metal hydride batteries self-discharge quickly, which means they lose juice just sitting in the drawer. In fact, they lose about 25 percent of their power each month with a shelf life of two to three months: not the best choice for an emergency flashlight battery. There are batteries on the market that recharge using the sun and come with their own photovoltaic panel to harness energy from sunlight. Some flashlight bodies have a built-in photovoltaic panel and need to be simply set in direct sunlight to recharge. Depending on where you live, rechargeable batteries such as these might be a worthy investment.
Good 01' Alkaline Batteries
Confused about the many choices of batteries yet? I go to the discount or grocery store and buy the brand name batteries they have on the shelf, usually the alkaline type, which were invented more than forty years ago by a scientist named Lew Urry. More than 2 billion alkaline batteries are sold each year, dwarfing all other battery sales combined. On average, alkaline batteries have a shelf life of five to seven years. Look at the date printed on the batteries at the store and buy the ones with the latest date. After seven years, the batteries might still retain 80 percent of their juice but they won't perform as long or as well, so, in other words, use at your own risk and get used to rotating your battery stock each year. Regular alkaline batteries are available in common sizes and are used in all sorts of battery-operated devices, so rotating your stock and buying fresh ones shouldn't be a big deal; another plug for simple flashlights that use simple batteries without the bells and whistles of the specialty lights.
Store your batteries like you should store your flashlight: at room temperature, out of direct sunlight, and in a dry area—NOT in the refrigerator. Moisture absorbed by batteries from the tuna surprise casserole can cause the battery to not work and/or drastically reduce their shelf life and performance. Keep extra batteries in their original package for storage. Contact with other metal items, including the batteries themselves, can short-circuit the battery so don't carry loose batteries in a pocket, purse, or pack. As to which brand of batteries to purchase, Consumer Reports found very little difference between brand name battery companies and their product despite the commercials. I have found that Duracell batteries seem to work and hold up better in the cold than Energizers, but to each his own. In years past alkaline batteries contained mercury, which made them bummers to dispose of. In 1996, however, Congress banned mercury from all household batteries. Now they can be safely thrown away in typical household trash when they poop out, or you can recycle them at some electronic stores such as Radio Shack.
More expensive lithium batteries, now commonly available at most grocery stores, have up to a fifteen-year shelf life. That's where the advantage ends, however, for our emergency flashlight motives. Lithium batteries are typically reserved for devices that require a lot of power in a short amount of time such as MP3 players, digital cameras, and CD players. Just in case you're getting testy and want to argue the lithium battery point, call the Energizer battery company 1–800 number available on the Internet like I did. They will tell you that other than the longer shelf life, lithium batteries for flashlights are, and I quote, "a waste of money."
Spare alkaline batteries carried in my survival kit for one year give approximately four hours of light with the first three hours being the brightest. The last hour of light is marginal but still useful for close-up household tasks. You should rotate your batteries in the flashlight as well as your spares every year, whether you have put them to use or not. I bind my spares together with brightly colored tape and write the month and year they were purchased in permanent marker on the tape. This takes the guesswork out of when you should rotate the little gems.
The ends of batteries corrode quickly, even in the arid Southwest, so get in the habit of inspecting your spares a few times a year. If you live in a wet climate, plan on rotating them more frequently. Although in a pinch the corrosion can be scraped off the ends, it's a safer bet to replace them entirely. As a bonus, batteries—even AA batteries—can be placed end to end and used in conjunction with superfine steel wool to start a fire.
Light Sticks
Light sticks are just one application of an important natural phenomenon—luminescence. Generally speaking, luminescence is any emission of light that is not caused by heating. Among other things, luminescence is used in televisions, neon lights, and glow-in-the-dark stickers. It's also the principle that lights up a firefly and makes some rocks glow after dark.
Since their inception twenty-eight years ago, chemical light sticks have made their way well into urban culture and are available at many camping and big box stores. Nearly every little kid during Halloween becomes a glowing example of the power of two chemicals mixed together. Light sticks are used for a variety of applications from scuba diving to the rave dance scene. I use them to mark base camp shelters in the winter woods and desert caches of water at night for weary travelers on my field courses.
While not super cheap (two to four dollars apiece), light sticks are reasonably priced and put out enough light to get a range of close-quarters tasks accomplished with minimum hassle. These bulbless, batteryless wonders are light and portable and, dare I say it, more or less kidproof. The chemical reaction that takes place within the light stick generates absolutely no heat so parents don't have to worry about junior lighting the drapes on fire, scorching the pet cat, or otherwise burning down the house. This point should not be taken lightly as, aside from flashlights, finding a decent lighting alternative that is also kid-safe is a challenge.
How light sticks emit their glow is fairly simple. They consist of a small glass vial, or activator, filled with a hydrogen peroxide solution. This glass vial is housed inside the middle of a larger plastic vial, containing a phenyl oxalate ester and dye solution. The last two chemicals make up the majority of the light stick's guts. By bending the plastic stick, the glass vial breaks open, and the two solutions flow together. The chemicals immediately react to one another in a process called
chemiluminesence
and the atoms begin emitting light. The particular dye used in the chemical solution gives the light a distinctive color, of which there are several to choose from. The white ones put out the most light, next yellow, and then take what you can get.
Depending on which compounds are used, the chemical reaction may go on for a few minutes or many hours. By heating the solutions, the extra energy will accelerate the reaction, and the stick will glow brighter, but for a shorter amount of time. Put an activated light stick in boiling water for a few minutes and see for yourself. If you cool the light stick, the reaction will slow down, and the light will dim. If you want to preserve your light stick for the next day, put it in the freezer or outside in cold weather—it won't stop creating light but it will drag out the reaction considerably. However, the light emitted at this point is not something you'd choose to read a book by.
LIGHT MY FIRE! USING BATTERIES
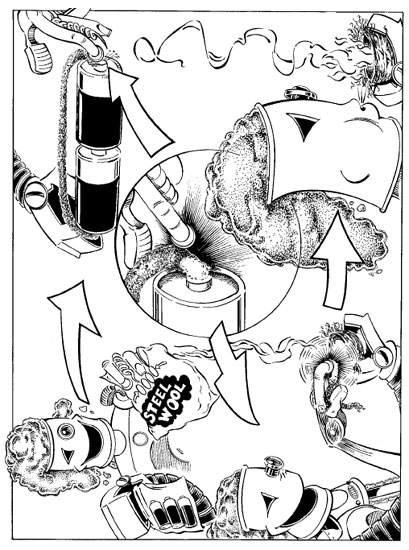
AND STEEL WOOL TO MAKE FIREFor this method, avoid using batteries smaller than the AA size. Box-shaped batteries that feature both terminals on the same battery can also be used. Put two batteries together end to end, the nipple of one touching the base of the other. Hold one end of the steel wool to the base end, and touch the other end of the steel wool to the nipple end. Then put the glowing result into a suitable tinder bundle and gently blow it into a flame. If you wish to use a car battery, and it's still in the car, open the hood and let the engine compartment air out. Volatile gases from batteries can explode so use caution and common sense. Don't use a longer or thicker piece of steel wool than necessary to reach the opposing battery terminal as it will require more voltage from the battery. Also, keep the ends of the steel wool fairly loose yet neat, not tightly compressed. Take it away, Robbie!
Candles
Candle manufacturer surveys report that 96 percent of all candles are purchased by women and are used in seven out of ten U.S. households. With more than 350 commercial, religious, and institutional manufacturers of candles in the United States alone, as well as countless small craft producers, it's no wonder that retail sales of candles in the United States is estimated at $2 billion per year, excluding the sales of candle accessories. Major candle manufacturers may offer up to 2,000 varieties of candles in their product line, including tapers, straight-sided dinner candles, columns, pillars, votives, wax-filled containers, and an assortment of specialty candles in a variety of sizes, fragrances, and colors.
