Why Is Milk White? (10 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho
All of these reactions are sped up by acids and by having more ions in the water, so it conducts electricity better, so that the iron and oxygen can exchange electrons. Adding salt to the water makes the iron corrode more quickly, but adding an acid makes it corrode even faster than that.
Simple water filters just block large particles and let tiny water molecules go on through. You can see this effect when you rinse vegetables in a strainer or colander. The vegetables are the large “particles” that are blocked.
A paper towel will block smaller particles. You can use paper as a coffee filter, to keep the coffee grounds in the paper while the water and other small molecules (the ones that give coffee its flavor and color) go on through.
If you want to get rid of those other small molecules, you might use an activated charcoal filter. Charcoal is very good at absorbing small molecules that color and flavor water. This action is not actually filtering; it is absorbing.
Once the charcoal has absorbed a lot of those small molecules, it gets full and can't absorb any more. To reactivate it, you just heat it up. The small molecules boil off, and the charcoal is ready to absorb more again.
Paper filters and charcoal filters won't block tiny things like bacteria. For that, the holes in the filter must be very tiny. But that
means that the filter has to be very big, because even the tiny water molecules will be blocked most of the time. An alternative is to use a lot of pressure to force the water through the filter.
One type of filter that uses a lot of pressure to force water through tiny pores is a
reverse osmosis
filter. In normal osmosis, a filter sits between pure water and salt water. The water can go through the filter in either direction, but the salt ions are too big to go through the filter. This means that more molecules end up on the salt side of the filter. To reverse this, a large pressure is used on the salt side of the filter to force the pure water through. So instead of water going from the pure side to the salty side, the direction is reversed, and you get pure water out.
In the discussion of how washing soap works (
page 28
), you learned how calcium and magnesium could attach to two soap molecules, making a large molecule that was too big to stay dissolved in the water. This large molecule is what forms soap scum, and it sticks to skin, hair, and fabric, gluing dirt onto them.
To make it easier for soap to work, calcium and magnesium ions are removed from the water. One way to do this is to add a chemical to the water that combines with the calcium and magnesium and makes them unavailable to combine with the soap. Citric acid will do this. So will sodium carbonate, which is why it is called
washing soda.
Another way to remove the calcium and magnesium ions is to use a water softener. A water softener runs the water through a tank full of tiny plastic beads. These beads have a negative charge and collect positive ions.
At first the beads have sodium ions around them. But sodium ions only have a charge of +1. Calcium and magnesium have charges of +2, so they are attracted to the negatively charged plastic
beads more than the sodium ions are. The two types of ions then exchange places. That is why the plastic the beads are made of is called an
ion exchange resin.
At some point all of the sodium ions have been exchanged for calcium and magnesium ions, and the plastic beads have to be recharged with sodium ions. To do that, the water softener gives them an overwhelming supply of sodium ions by bathing them in very salty water. Even though the calcium and magnesium ions are more positive, there are so many sodium ions that the reaction goes into reverse.
Once the plastic beads are again coated with sodium ions, the salty water, which now has lots of calcium and magnesium ions in it, is flushed away, and the water-softening cycle repeats.
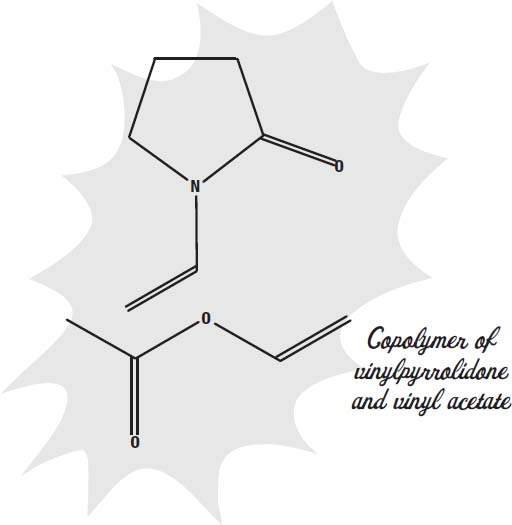
Glue. Actually, vinyl acetateâwhat common white glue is made ofâis one of the common ingredients, but other ingredients are in hairspray to modify the glue to be just right for holding hair.
Hairspray needs to start out as a bunch of tiny droplets of glue that run down strands of hair until they come to the place where two strands cross. The drop stops there and dries into a thin film of glue that holds the two strands together. Repeat this a few thousand times, and your hair will stay in place all day.
But hairspray also has to be a glue that washes out of your hair easily when you shampoo and can't remain sticky or become sticky on a humid day or in rain or fog. So usually a second polymer is added, one that is not water soluble. Hairspray is thus made of
copolymers,
which just means two kinds of glue.
The second polymer is often crotonic acid or vinylpyrrolidone.
The polymers are dissolved in alcohol and a solvent propellant such as butane or dimethyl ether.
Plasticizers make the glue more flexible. These might be silicones or fatty acid esters (fatty acids bound to alcohols) such as triethyl citrate. There are also usually some additives that are mainly
used to keep the can from rusting inside, such as aminomethyl propanol or cyclohexylamine.
Hairsprays may also include emollients (skin softeners) such as cyclop entasyloxane. Dimethyl stearamine is sometimes added to reduce static electricity (by making the hair surface conduct electricity better). Many fragrant chemicals may also be added, such as linalool, limonene, butylphenyl methylpropional, amyl cinnamal, hexyl cinnamal, citronellol, or geraniol. These are citrus and floral scent molecules. Some hairsprays contain sunscreens such as ethylhexyl methoxycinnamate.
The glue in hairspray connects strands of hair together, so that they don't slide past one another. But to make hair really stiff so that it will hold its shape into elaborate spikes and other shapes, some people use egg white, gelatin, or sugar water as the glue and then spray hairspray over it when it has dried to keep it from feeling sticky.
For much the same reasons anything is slimy. Slimy is the word we use for the feel of things scientists call
hydrocolloids
. These are made of water and molecules that are too big to dissolve but too small to settle to the bottom of the water.
Hydrocolloids don't
always
feel slimy. Gelatin is a hydrocolloid that is generally made with so much protein that it is almost solid. But with less protein, it feels slimy too. Soak some grains of unflavored gelatin in water, and the grains feel slimy.
Pectinâthe starch-like molecule we add to fruit juice to make jellyâfeels slimy. White glue feels slimy, especially if you add borax to make slime. (See the project “Fun with Boron” on
page 192
.) The slime that snails make is also a hydrocolloid.
If you remove some water from a hydrocolloid, it usually gels. That is, it becomes more solid. This characteristic is useful in a hair product, since it helps the product stay in the hair and not run down the back of your neck. It is solid enough to hold in your hand without dripping but becomes more liquid as you rub it into your hair.
How a hydrocolloid reacts to the stress of working it into your hair depends on the nature of the particles in the water. Corn starch hydrocolloids are famous for becoming thick and almost solid when you hit them suddenly, while being almost completely liquid if you move your finger through them slowly. Hair gel is designed with smaller particles that have the opposite behavior. Hair gel thins when stressed, so it can be worked into the hair.
Like hairspray, hair gel is made of polymers. The polymers are water soluble and tend to have positively charged areas that are attracted to the negatively charged areas of the hair strand.
As the gel is applied, the long strands of the polymers wrap around the hair strands and bind them together. When the gel dries, the polymers shrink around the strands of hair, pulling them tightly together.
Hair gels usually contain alcohol so they dry faster. Other ingredients include plasticizers (like the ones in hairspray) that keep the polymers soft and elastic, and usually some type of fragrance. Sunscreens are another common additive.
The main gelling agent is often a vegetable gum, such as guar gum. Other vegetable gums, such as marshmallow and aloe vera, are sometimes used. For extra hold and support for elaborate hairstyles, acrylic plastic polymers (similar to floor polish and white glue) are used. Humectants (molecules that absorb water from the air) are used to keep the gel from completely drying out and feeling crusty. The simplest ones are just sugars, such as agave nectar.
Silicones. Although that particular brand name includes shampoos and hairsprays, only conditioners and detanglers are talked about in this section. To allow wet hair strands to easily slide past one another, conditioners and detanglers use two strategies.
First, they have ingredients that make the tiny scales on the hair shaft lie down, so they don't have rough surfaces that grip other hair shafts. Citric acid is sometimes used for this purpose.
Second, they coat the hair shaft with slippery oils and silicones to make the hair shafts glide across one another easily during combing and brushing.
In Biosilk, cyclomethicone and dimethicone are the slippery silicone oils that help the strands untangle.

These are often used in other products as carrier fluids in hairsprays or as skin protectants to act as a moisture barrier and prevent chafing.
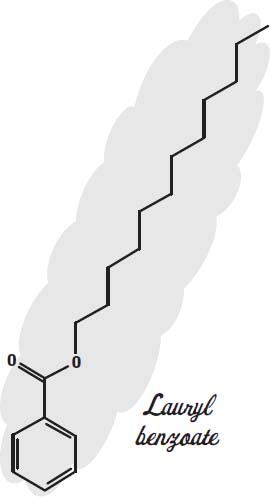
Conditioners that you leave in the hair (instead of rinsing out) will contain mostly silicones and alcohol. To make the silicones feel less greasy, alkyl benzoates such as lauryl benzoate are used.
Alkyl benzoates are lubricants themselves and also serve as solvents to carry sunscreens, and they are emollients, keeping the skin from drying out. Alkyl
benzoates are esters, compounds that result when an alcohol reacts with an acid, in this case a fatty acid from vegetable oil. The alcohol end (the hexagonal benzyl alcohol at the bottom of the drawing) modifies the fatty end, so that it can join together oils and silicones and also help retain the fragrance molecules.
Because we want the makeup to do so many things.
Many of the ingredients are dyes and pigments to change the color of the skin or hair. Then something is needed to make the colors easy to apply to the skin, so carriers and propellants are added. Sunscreens are also needed to protect the skin from the sun, emollients and humectants to keep the skin from drying out, and preservatives to give the makeup longer shelf life.
Chemists could use oils in the makeup, but those can cause acne, so they modify the oils by making esters out of the fatty acids to get an oil-free makeup that does not cause the skin to break out. (See the lauryl benzoate molecule on the previous question for an example.)
Some makeups have both fat-soluble ingredients and watersoluble ingredients. To get these to mix and not separate like oil and vinegar dressing,
emulsifiers
are added to make something closer to a mayonnaise out of the watery and oily parts. An emulsifier is like a detergentâit is a molecule with one end that sticks to oil while the other end sticks to water.