Why Is Milk White? (15 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho
You won't explode. Like any other dust, gunpowder is unpleasant to breathe in. You will cough and sneeze as your body tries to get rid of it.
The charcoal in black powder is not toxic. Sulfur is also fairly safe in the quantities you would inhale. Potassium nitrate is a lung irritant. None of the ingredients would be likely to be fatal if inhaled with a single breath, but it would not be fun. In general, I would recommend against inhaling powders of any sort.
Modern smokeless powders are not really powders and are much harder to inhale. Nitrocellulose might give you a headache. Nitroglycerin, on the other hand, affects the blood vessels, dilating them. This lowers blood pressure (which is why people with heart disease take nitroglycerin pills).
The first fireworks were made in China over a thousand years ago. They used the newly invented black powder as a propellant for rockets and as a noisemaker.
You need two things to make an exploding firecracker from black powder: the powder and a closed container. Black powder by itself will just burnâit won't explode. But if it is in a closed container, pressure will build up as it burns until the container bursts all at once. The sudden release of all the hot gas makes a loud noise. Just like when you close a door, how much noise it makes depends on how fast you close it.
Some fireworks explode without a closed container. These don't use black powder. Instead, they use high explosives that burn faster than the speed of sound. This is called
detonating
, and it breaks the sound barrier just like a jet making a sonic boom.
Some simple fireworks, like sparklers, are safe enough to make at home. They are basically gunpowder recipes with added iron or
aluminum to make the sparks and some sugar or starch in water to make a paste that sticks on the wire.
Firecrackers are gunpowder packed into cardboard tubes that act as the closed container needed to make noise. A fuse is often just gunpowder wrapped in tissue paper. The fuse burns slowly enough to let you get away from the firecracker before the gunpowder in the cardboard tube ignites and explodes.
Rockets are also often simple cardboard tubes filled with gunpowder, but with one end capped with a clay nozzle instead of being completely closed. When lit, the powder burns instead of exploding, and the hot gas comes out the nozzle. The force that it creates pushes the rocket away.
Mortars are small cannons made of cardboard tubes. A complex explosive device called a
shell
is lit and dropped into the tube. The bottom of the shell is a firecracker that explodes. This pushes the rest of the shell out of the tube into the air, where it can explode high above the spectators. It often has little bits of gunpowder called
stars.
These create the flower shapes as they burn brightly in the air after the shell explodes. The stars can have additives that make them burn brighter or burn in different colors.
We hear sounds when air molecules push on our eardrums. The harder they push, the louder the sound.
To create sounds, air must be pushed. The faster the air is pushed, the more pressure that is created, since the air has less time to get out of the way.
If you push on the air with your hand, you can feel the pressure of the air on your face, but you won't hear any sound because your hand is moving too slowly. To make a sound, the air must move faster than it can get away. You can do this by clapping your hands together. That traps air between our hands, increasing the pressure. The air molecules can't get away fast enough, so they all get crowded together.
When an explosive is set off, it produces a lot of molecules of gas in a very short time. These molecules bounce into air molecules and make sound waves. The faster the explosive burns, the louder the sound waves will be.
A firecracker moves air faster than your clapping hands can move air, so it sounds louder than hand clapping. Dynamite explodes faster than gunpowder, so it makes an even louder sound.
Grenades are small devices that contain explosives and are launched or thrown so that they explode away from the person who threw them.
Hand grenades are thrown by hand. Other grenades have launchers. Some launchers attach to rifles, while others are carried by themselves, such as rocket propelled grenades, or RPGs.
Explosive grenades often have steel cases that are cut so that when the device explodes the bits of steel will act like bullets. These are called
fragmentation grenades.
Others called
concussion grenades
merely explode; they are meant to stun people.
Grenades use several chemical reactions to work. When the grenade leaves the thrower's hand, a spring pushes a pin down on a percussion cap. That cap explodes and lights a four-second fuse. That fuse eventually sets off a detonator, which then sets off the main explosive charge.
Not all grenades are actually explosive. Some are designed to set fires. These are called
incendiary grenades.
Others release smoke or tear gas or just make a loud noise or flash of light.
Several compounds can be used. A contact explosive made from phosphorus, sulfur, and potassium chlorate will explode when hit with the small hammer in a cap gun. This is similar to the mixture
used in “strike anywhere” matches. An early formula (from 1926) used potassium chlorate, sulfur, and antimony. You can tell if a cap has sulfur in it by the smell it makes (like a struck match) when it explodes.
As mentioned on page 105, contact explosives are those that are easy to set off. They're made from compounds, such as potassium chlorate or potassium perchlorate, that are very good oxidizing agents. Fuels that are very easily oxidized, such as sulfur, phosphorus, or antimony, are added to the powerful oxidizer to create an explosive sensitive enough to be set off by the force of the hammer in the cap gun.
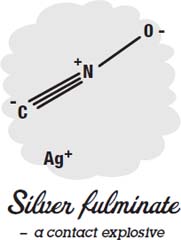
Other exploding toys, such as the “bang snaps” novelty fireworks, use a different contact explosive, a high explosive called silver fulminate. A tiny amount of silver fulminate is added to some sand, which is then wrapped in thin paper. When thrown at something, the explosive makes a loud bang, but the sand absorbs most of the energy, so no damage is done.
Fulminates are made by reacting gold, silver, mercury, or platinum with ammonia or nitric acid. Those metals don't react easily, and the compounds they create break apart easily, releasing energy. Most contact explosives use compounds that break apart easily and release energy. Other examples are ammonium triiodide, triacetone tri-peroxide, lead azide, and lead styphnate.
One part of what makes hot things a certain color is just how hot they are. You can see this when you look at the wires in a toaster glowing from dull red to bright orange as they heat up. You can see the color of an incandescent light change as
you move a dimmer switch from low to high. The dull orange of the lowest setting becomes the white hot of the highest setting.
In a flame from burning gas or alcohol, there are often two main colors, orange and blue, which are produced through different mechanisms. The orange comes from particles of unburned soot that glow from the heat. Their color is determined by the temperature in what is called
blackbody radiation
. Red is the coolest flame, then orange, yellow, white, and finally blue.
Blue color would indicate a temperature of over 10,000 degrees Celsius if the sooty part of the flame were that color. However, the blue part of the flame we normally see gets its color from a different mechanism. Electrons in atoms and molecules can be excited into higher energy levels as the gas or alcohol burns. These electrons can emit that energy as light when they fall back down to their normal energy levels. The color of light they emit indicates what molecules they are, since each molecule has a different energy level. The blue comes mainly from a molecule made from one carbon atom bonded to one hydrogen atom. Some of the light in the greenish-blue portion of the flame comes from a molecule made of two carbon atoms.
As discussed in the answer to the previous question, the blue part of a gas flame comes from electrons falling from excited high energy levels to their normal lower levels. The same mechanism can be used to get colors other than blueâjust add some atoms or molecules that have different energy levels.
The easiest color to add to a flame is bright yellow, from adding sodium. Ordinary table salt will work just fine. You can soak a paper towel in salt, let it dry, and then burn it. Or you can just sprinkle some salt into a gas flame.
Copper is famous for its blue- and green-colored compounds. If you put some copper compounds into a flame, you get pretty blues and greens. Lithium salts make red flames, and strontium
salts make even brighter red flames. Barium salts make blue-green flames, and potassium makes violet flames. When you want to make fireworks with sparks of different colors, you can add these compounds to the burning materials.
Some burning materials make colors by other mechanisms. Magnesium burns with a bright white light, because it is hot enough to get into the white region of the blackbody radiation curve. Similarly, orange colors in fireworks are often made from glowing balls of carbon, hot enough to glow orange.
Plasma is a gas that is so hot that the molecules or atoms lose some of their electrons. On Earth, the most common forms of matter are solid, liquid, and gas. But in the universe as a whole, the most common form of matter is plasma.
The sun is a big ball of plasma. Lightning is a plasma. The electric sparks you get from static electricity are made of plasma. There is a plasma inside every glowing fluorescent light tube and every neon light.
Plasmas consist of electrons and the positively charged atoms the electrons were stripped from. These atoms that are missing electrons are called ions, and we say that the gas that has become a plasma has been
ionized.
Gases that have only a small percentage of their atoms stripped of electrons are said to be weakly ionized. When more of the atoms are affected, the plasma is said to be highly ionized.
Most flames are weakly ionized plasmas. Sparks and lightning can be highly ionized.
Because electrons carry a negative charge and stripped atoms carry a positive charge, plasmas can conduct electricity. Gases do not conduct electricity.
Plasmas can emit colors just like flames do. The color of a neon light is due to excited electrons falling back into their normal energy levels, emitting light to lose the extra energy. Other
plasmas emit other colors, since their atoms or molecules have different energy levels. Helium plasmas are pink. Plasmas made from sodium vapor are the characteristic yellow of sodium.
Most of the volcanoes made for grade-school science fair projects are made using a baking soda and vinegar reaction to create a foam to represent the lava. Dishwashing soap is often added, along with food coloring, to get a red or orange appearance.
Baking soda is a salt made from a strong base and a weak acid. The strong base is sodium hydroxide, and the weak acid is carbon dioxide dissolved in water, the same thing you drink in soda water.
When a stronger acid (such as vinegar) is added to the baking soda, it replaces the weak acid. Since the weak acid is the same fizzy water in sodas, it fizzes in the volcano and makes tiny bubbles. The dishwashing soap helps these bubbles form a foam instead of just popping when they reach the surface.
If you like your volcanoes with real flames and sparks, you can use ammonium dichromate. A small pile of the compound can be lit with a match but should be done
with adult supervision, outside in a fire pit, barbecue, or fire-safe location.
It then burns with pretty red flames and orange sparks, and the ash expands, producing a typical volcano cone.
The ammonium dichromate is not actually burning. It is undergoing thermal decomposition. The atoms in the molecule are rearranging themselves to be in a lower energy state. If the compound is heated, it breaks down into green chromium oxide ash, water vapor, and nitrogen gas.
MAKING OXYGEN
Adult
supervision
required
Materials
Protective goggles
1 packet active dry yeast (available at grocery stores)
2-liter soda bottle, empty and clean
Hydrogen peroxide, 3 percent solution (available at drugstores and pharmacies)
Balloons
Candle
Bamboo skewer (optional)
Matches (optional)

You probably have everything you need to fill a balloon with pure oxygen. All it takes is some hydrogen peroxide from the medicine cabinet and some yeast from the kitchen.
All oxygen-breathing creatures make hydrogen peroxide as an inevitable byproduct of respiration. Since it can damage the cell if it is not destroyed, most cells have an enzyme called
catalase
that breaks hydrogen peroxide down into water and oxygen. That is why hydrogen
peroxide bubbles when you put it on a cut or scrape. Your own body has produced catalase, and that is breaking up the peroxide.
Yeast cells also breathe air and use the oxygen to burn sugar to get their energy. So they also produce hydrogen peroxide that they need to get rid of by using the catalase enzyme. So if you put some yeast in a bottle and pour in some hydrogen peroxide, you get bubbles of oxygen. The reaction is
exothermic,
meaning it generates heat, and you can feel the bottle get warm in your hand as the reaction takes place.
Pour the contents of a packet of active dry yeast into an empty two-liter soda bottle. Pour up to a couple inches of the hydrogen peroxide into the bottle. Three percent hydrogen peroxide, the kind you use for first aid, works fine. Be sure not to fill the bottle too high.

Place a balloon over the top of the bottle and watch it inflate as it fills with oxygen. The reason you had to be careful not to fill the bottle too high was so the bubbles won't flow over of the top and into the balloon. You may still have to shake or tap the bottle to prevent the bubbles from rising too high.
Once you have a balloon full of oxygen, you can start to have fun. For safety reasons,
always wear protective goggles
for this next part of the experiment.
First, see what happens when you blow up a balloon the normal way, using air from your lungs, and then aim it at a lit candle. Just like what happens on a birthday cake, the candle simply goes out. Now see what happens when you try to blow the candle out using the balloon full of pure oxygen.

See the video at
http://youtu.be/ke6v-QYM4kAM
The candle doesn't go out. Instead, it gets brighter. Look at how the camera adjusted to the extra brightness by making the rest of the picture dimmer.
You can use the same technique to make oxygen in a water glass, and then try to pop a bubble of oxygen with a hot coal on the end of a stick. (You can create one easily by lighting a bamboo skewer on fire then blowing it out, turning the end into a glowing-red coal.) When the bubble pops, a small explosion
happens as the oxygen causes the hot coal to burst into flames. Of course,
you'll need protective goggles
for this experiment as well.

See the video at
http://youtu.be/FPaDHie3tM8