In Search of Memory: The Emergence of a New Science of Mind (32 page)
Read In Search of Memory: The Emergence of a New Science of Mind Online
Authors: Eric R. Kandel
Tags: #Psychology, #Cognitive Psychology & Cognition, #Cognitive Psychology
By studying living sensory and motor cells in culture we had reduced our behavioral system sufficiently to address these questions. We had located a critical component of long-term memory in the synaptic connection between only two cells. Now we could use the techniques of recombinant DNA to ask: Do regulatory genes switch on and maintain long-term strengthening of this connection?
AT ABOUT THIS TIME, I BEGAN TO RECEIVE FORMAL RECOGNITION
for my work. In 1983 I shared with Vernon Mountcastle the Lasker Award in basic medical sciences, the most important scientific recognition awarded in the United States, and I received my first honorary degree, from the Jewish Theological Seminary in New York. I was thrilled that they would even know of my work. I suspect they learned of it from my colleague Mortimer Ostow, one of the psychoanalysts who first stirred my interest in psychoanalysis and the brain.
My father had died by then, but my mother came to the graduation ceremony and, in his introductory remarks, Gerson D. Cohen, the chancellor of the seminary, referred to my having received a good Hebrew education at the Yeshivah of Flatbush, an acknowledgment that filled my mother’s Jewish heart with pride. I think the recognition that her father, my grandfather, had tutored me well in Hebrew may have meant more to her than the distinction of the Lasker Award a few months later.
I
n 1985 I finally began to apply the insights I had gained from night science—from months of thinking about the proteins that regulate gene expression—to a daytime framework for working on gene expression and long-term memory. This thinking had become more focused with the arrival at Columbia of Philip Goelet, a postdoctoral student who had trained with Sydney Brenner at the Medical Research Council Laboratory in Cambridge, England. Goelet and I reasoned as follows: long-term memory requires that new information be encoded and then consolidated, put into more permanent storage. With the finding that long-term memory requires the growth of new synaptic connections, we had gained some insight into what form this more permanent storage took. But we still did not understand the intervening molecular genetic steps—the nature of memory consolidation. How is a fleeting short-term memory converted to a stable long-term memory?
In the Jacob-Monod model, signals from a cell’s environment activate gene regulatory proteins that switch on the genes encoding particular proteins. This led Goelet and me to wonder whether the crucial step in switching on long-term memory in sensitization might involve similar signals and similar gene regulatory proteins. We wondered if the repeated learning trials required for sensitization are important because they send signals to the nucleus, telling it to activate regulatory genes that encode regulatory proteins that in turn switch on the effector genes needed for the growth of new synaptic connections. If so, the consolidation phase of memory might be the interval during which the regulatory proteins switch on effector genes. Our thinking would provide a genetic explanation for the finding that blocking the synthesis of new protein during a critical period—that is, during and shortly after learning—blocks both the growth of new synaptic connections and the conversion from short- to long-term memory. In blocking protein synthesis, we reasoned, we were actually preventing the expression of the genes that initiate protein synthesis essential for synaptic growth and long-term memory storage.
We summarized our views in “The Long and Short of Long-Term Memory,” a conceptual review published in 1986 in
Nature
. In this paper, we proposed that if gene expression was required to convert short-term memory at a synapse into long-term memory, then the synapse stimulated by learning somehow had to send a signal to the nucleus telling it to turn on certain regulatory genes. In short-term memory, synapses use cyclic AMP and protein kinase A inside the cell to call for the release of more neurotransmitter. Goelet and I hypothesized that in long-term memory this kinase moves from the synapse to the nucleus, where it somehow activates proteins that regulate gene expression.
To test our hypothesis, we would have to identify the signal sent from the synapse to the nucleus, find the regulatory genes activated by the signal, and then identify the effector genes switched on by the regulator—that is, the genes responsible for the new synaptic growth underlying long-term memory storage.
THE SIMPLIFIED NEURAL CIRCUIT WE HAD CREATED IN TISSUE
culture—a single sensory neuron connected to a single motor neuron—gave us a complete biological system in which to test these ideas. In our culture dish, serotonin acted as an arousal signal initiated by sensitization. One pulse—the equivalent of one shock, one training trial—alerted the cell that a stimulus was of momentary, short-term interest, while five pulses—the equivalent of five training trials—signaled a stimulus of lasting, long-term interest. We found that injecting a high concentration of cyclic AMP into a sensory neuron produced not only a short-term but also a long-term increase in the strength of the synapse. We now collaborated with Roger Tsien at the University of California, San Diego and used a method developed by him that allowed us to visualize the location of the cyclic AMP and protein kinase A in the neuron. We found that whereas a single pulse of serotonin increases cyclic AMP and protein kinase A primarily at the synapse, repeated pulses of serotonin produce even higher concentrations of cyclic AMP, causing protein kinase A to move into the nucleus, where it activates genes. Later studies found that protein kinase A recruits another kinase, called MAP kinase, which is also associated with synaptic growth and also migrates to the nucleus. Thus we confirmed our idea that one of the functions of repeated sensitization training—why practice makes perfect—is to cause the appropriate signals in the form of kinases to move into the nucleus.
Once in the nucleus, what do these kinases do? We knew from recently published studies of non-neuronal cells that protein kinase A can activate a regulatory protein called CREB (cyclic AMP response element-binding protein), which binds to a promoter (the cyclic AMP response element). This suggested to us that CREB might be a key component of the switch that converts short-term facilitation of synaptic connections to long-term facilitation and the growth of new connections.
In 1990, joined by two postdoctoral students, Pramod Dash and Benjamin Hochner, we found that CREB is present in the sensory neurons of
Aplysia
and is indeed essential to the long-term strengthening of synaptic connections underlying sensitization. By blocking the action of CREB in the nucleus of a sensory neuron in culture, we prevented long-term, but not short-term, strengthening of these synaptic connections. This was astonishing: blocking this one regulatory protein blocked the entire process of long-term synaptic change! Dusan Bartsch, a creative and technically brilliant postdoctoral fellow, later found that simply injecting CREB that had been phosphorylated by protein kinase A into the nucleus of sensory neurons was sufficient to turn on the genes that produce long-term facilitation of these connections.
Thus, even though I had long been taught that the genes of the brain are the governors of behavior, the absolute masters of our fate, our work showed that, in the brain as in bacteria, genes also are servants of the environment. They are guided by events in the outside world. An environmental stimulus—a shock to an animal’s tail—activates modulatory interneurons that release serotonin. The serotonin acts on the sensory neuron to increase cyclic AMP and to cause protein kinase A and MAP kinase to move to the nucleus and activate CREB. The activation of CREB, in turn, leads to the expression of genes that changes the function and the structure of the cell.
In 1995 Bartsch found that there are in fact two forms of the CREB protein, much as the model of Jacob and Monod might have predicted: one that activates gene expression (CREB-1), and one that suppresses gene expression (CREB-2). Repeated stimulation causes protein kinase A and MAP kinase to move to the nucleus, where protein kinase A activates CREB-1 and MAP kinase inactivates CREB-2. Thus long-term facilitation of synaptic connections requires not only a switching on of some genes, but also the switching off of others (figure 19–1).
As these exciting findings were emerging in the laboratory, I was struck by two things. First, we were seeing the Jacob-Monod model of gene regulation applied to the process of memory storage. Second, we were seeing Sherrington’s discovery of the integrative action of the neuron carried to the level of the nucleus. I was amazed by the parallels: on the cellular level, excitatory and inhibitory synaptic signals converge on a nerve cell, while on the molecular level, one CREB regulatory protein facilitates gene expression and the other inhibits it. Together, the two CREB regulators integrate opposing actions.
Indeed, CREB’s opposing regulatory actions provide a threshold for memory storage, presumably to ensure that only important, life-serving experiences are learned. Repeated shocks to the tail are a significant learning experience for an
Aplysia
, just as, say, practicing the piano or conjugating French verbs are to us: practice makes perfect, repetition is necessary for long-term memory. In principle, however, a highly emotional state, such as that brought about by a car crash, could bypass the normal restraints on long-term memory. In such a situation, enough MAP kinase molecules would be sent into the nucleus rapidly enough to inactivate all of the CREB-2 molecules, thereby making it easy for protein kinase A to activate CREB-1 and put the experience directly into long-term memory. This might account for so-called flashbulb memories, memories of emotionally charged events that are recalled in vivid detail—like my experience with Mitzi—as if a complete picture had been instantly and powerfully etched on the brain.
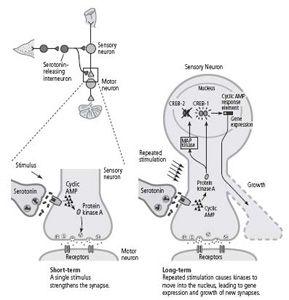
19–1
The molecular mechanisms of short- and long-term facilitation.
Similarly, the exceptionally good memory exhibited by some people may stem from genetic differences in CREB-2 that limit the activity of this repressor protein in relation to CREB-1. Although long-term memory typically requires repeated, spaced training with intervals of rest, it occasionally occurs following a single exposure that is not emotionally charged. One-trial learning was particularly well developed in the famous Russian memorist S. V. Shereshevski, who seemed never to forget anything he had learned following a single exposure, even after more than a decade. Usually, memorists have more restricted capabilities: they may be exceptionally good at remembering certain types of knowledge but not others. Some people have an astonishing memory for visual images, for musical scores, for chess games, for poetry, or for faces. Some Talmudic memorists from Poland can recall, from visual memory, every word on every page of the twelve volumes of the Babylonian Talmud as if that one page (out of several thousand) were in front of their eyes.
Conversely, a characteristic of age-related memory loss (benign senescent forgetfulness) is the inability to consolidate long-term memories. This defect of aging may represent not only a weakening of the ability to activate CREB-1, but also an insufficiency of signals to remove the braking action of CREB-2 on memory consolidation.
The CREB switch for long-term memory, like the cellular mechanisms of short-term memory, proved to be the same in several species of animals, indicating that it has been conserved through evolution. In 1993 Tim Tully, a behavioral geneticist working at the Cold Spring Harbor Laboratory in Long Island, New York, developed an elegant protocol for examining long-term memory of learned fear in the fly. In 1995 Tully joined forces with the molecular geneticist Jerry Yin, and together they discovered that CREB proteins are essential for long-term memory in
Drosophila
. As in
Aplysia
, CREB activators and repressors played critical roles. The CREB repressor blocked the conversion of short-term memory to long-term memory. Even more fascinating, mutant flies bred to produce more copies of the CREB activator had the equivalent of flashbulb memories. A few training trials in which a specific odor was paired with a shock produced only a short-term memory of fear of the odor in normal flies, but the same number of trials led to long-term memory of fear in the mutant flies. In time, it would become clear that the same CREB switch is important for many forms of implicit memory in a variety of other species, from bees to mice to people.
Thus, by combining behavioral analysis first with cellular neural science and then with molecular biology, we were able, collectively, to help lay the foundation of a molecular biology of elementary mental processes.
THE FACT THAT THE SWITCH FOR CONVERTING SHORT-TERM
memory to long-term memory is the same in a variety of simple animals that are learning simple tasks was heartening, confirming our belief that the core mechanisms of memory storage are conserved across different species. But it posed a considerable problem for the cell biology of neurons. A single sensory neuron has 1200 synaptic terminals and makes contact with about 25 target cells: gill motor neurons, siphon motor neurons, inking motor neurons, and excitatory and inhibitory interneurons. We had found that short-term changes occur in just some of those synapses and not others. That made sense, since a single shock to the tail or a single pulse of serotonin increases cyclic AMP locally, at a particular set of synapses. But long-lasting synaptic change requires gene transcription, which takes place in the nucleus and leads to the production of new proteins. One would expect the newly synthesized proteins to be shipped to all of the neuron’s synaptic terminals. Thus, unless some special mechanism in the cell limits the changes to specific synapses, all of the neuron’s synaptic terminals would be affected by long-term facilitation. If that were so, each long-term change would be stored at all the synapses of a neuron. This creates a paradox: How are long-term learning and memory processes localized to specific synapses?
Goelet and I thought about this question a great deal and outlined a scheme in our 1986 review in
Nature
that has become known as “synaptic marking.” We hypothesized that the transient modification of a given synapse produced by short-term memory would somehow mark that synapse. Marking would allow proteins to be recognized by and stabilized at that synapse.