In Search of Memory: The Emergence of a New Science of Mind (28 page)
Read In Search of Memory: The Emergence of a New Science of Mind Online
Authors: Eric R. Kandel
Tags: #Psychology, #Cognitive Psychology & Cognition, #Cognitive Psychology
To distinguish between the two spatially distinct functions of metabotropic receptors, Sutherland called the chemical messenger that binds to the metabotropic receptor on the outside of the cell the first messenger, and the cyclic AMP activated inside the cell to broadcast the signal the second messenger. He argued that the second messenger conveys the signal at the cell surface from the first messenger into the interior of the cell and initiates the cell-wide response (figure 16–2). Second-messenger signaling suggested to us that metabotropic receptors and cyclic AMP might be the elusive agents connecting the slow synaptic potential in sensory neurons to the enhanced release of glutamate and consequently to the formation of short-term memory.
In 1968 Ed Krebs at the University of Washington provided the initial insight into how cyclic AMP produces its widespread effects. Cyclic AMP binds to and activates an enzyme that Krebs called cyclic AMP-dependent protein kinase, or protein kinase A (because it was the first protein kinase to be discovered). Kinases modify proteins by adding a phosphate molecule to them, a process known as phosphorylation. Phosphorylation activates some proteins and inactivates others. Krebs found that phosphorylation can be readily reversed and thus can serve as a simple molecular switch, turning the biochemical activity of a protein on or off.
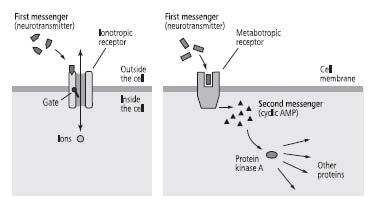
16–2 Sutherland’s two classes of receptors
. Ionotropic receptors (left) produce changes lasting milliseconds. Metabotropic receptors (e.g., serotonin receptors) act through second messengers (right). They produce changes that last seconds to minutes and are broadcast throughout the cell.
Krebs next went about finding out how this molecular switch works. He discovered that protein kinase A is a complex molecule made up of four units—two regulatory and two catalytic. The catalytic units are designed to carry out phosphorylation, but the regulatory units normally “sit” on them and inhibit them. The regulatory units contain sites that bind cyclic AMP. When the concentration of cyclic AMP in a cell increases, the regulatory units bind the excess molecules. This action changes their shape and causes them to fall off the catalytic units, leaving the catalytic units free to phosphorylate target proteins.
These considerations raised a key issue in our minds: Was the mechanism discovered by Sutherland and Krebs specific to the action of hormones on fat and muscle cells, or could it also involve other transmitters, including those present in the brain? If so, this would represent a previously unknown mechanism of synaptic transmission.
Here we were helped by the work of Paul Greengard, a gifted biochemist who was also trained in physiology and who had recently moved to Yale University from his former position as director of biochemistry at Geigy Pharmaceutical Research Laboratories. On the way to Yale, he stopped off for a year in Sutherland’s department. Realizing the importance of a potentially novel signaling mechanism in the brain, Greengard began in 1970 to sort out metabotropic receptors in the brains of rats. Now, a wonderful coincidence emerged that was to link Arvid Carlsson, Paul Greengard, and me on a scientific journey that would lead the three of us to Stockholm in 2000 to share in the Nobel Prize in Physiology or Medicine for signal transformations (transduction) in the nervous system.
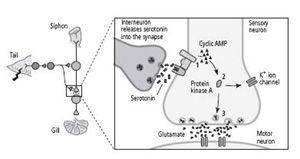
16–3 Biochemical steps in short-term memory
. A shock to the tail of
Aplysia
activates an interneuron that releases the chemical messenger serotonin into the synapse. After crossing the synaptic cleft, serotonin binds to a receptor on the sensory neuron, leading to production of cyclic AMP (
1
). Cyclic AMP frees the catalytic unit of protein kinase A (
2
). The catalytic unit of protein kinase A enhances the release of the neurotransmitter glutamate (
3
).
In 1958 Arvid Carlsson, a great Swedish pharmacologist, discovered dopamine to be a transmitter in the nervous system. He then went on to show that when the concentration of dopamine is decreased in a rabbit, the animal develops symptoms that resemble Parkinson’s disease. When Greengard started to explore metabotropic receptors in the brain, he started with a receptor for dopamine and found that it stimulates an enzyme that increases cyclic AMP and activates protein kinase A in the brain!
Based on these leads, Jimmy Schwartz and I discovered that cyclic AMP second-messenger signaling is also turned on by serotonin during sensitization. As we have seen, a shock to
Aplysia
’s tail activates modulatory interneurons that release serotonin. Serotonin, in turn, increases the production of cyclic AMP in the presynaptic terminals of the sensory neurons for a few minutes (figure 16–3). Thus it all came together: the increase in cyclic AMP lasts about as long as the slow synaptic potential, the increase in synaptic strength between the sensory and motor neurons, and the animal’s enhanced behavioral response to the shock applied to its tail.
THE FIRST DIRECT CONFIRMATION THAT CYCLIC AMP IS INVOLVED
in the formation of short-term memory came in 1976, when Marcello Brunelli, an Italian postdoctoral fellow, joined our lab. Brunelli tested the idea that when serotonin signals the sensory neurons to increase the concentration of cyclic AMP, the cells boost the amount of glutamate released from their terminals. We injected cyclic AMP directly into a sensory cell of
Aplysia
and found that it dramatically increased the amount of glutamate released and, therefore, the strength of the synapse between that sensory cell and motor neurons. In fact, injecting cyclic AMP simulated perfectly the increased synaptic strength brought about by applying serotonin to sensory neurons or a shock to the animal’s tail. This remarkable experiment not only linked cyclic AMP and short-term memory but also gave us our first insight into the molecular mechanisms of learning. Having begun to trap the basic molecular components of short-term memory, we could now use them to simulate memory formation.
In 1978 Jimmy and I began to collaborate with Greengard. The three of us wanted to know whether cyclic AMP produces its effect on short-term memory through protein kinase A. We pulled the protein apart and injected directly into a sensory neuron only the catalytic unit, which normally carries out phosphorylation. We found that this unit does exactly what cyclic AMP does—it strengthens the synaptic connection by enhancing the release of glutamate. Then, just to make sure we were on the right track, we injected an inhibitor of protein kinase A into a sensory neuron and found that it indeed blocked the ability of serotonin to enhance glutamate release. In finding that cyclic AMP and protein kinase A are both necessary and sufficient for strengthening the connections between sensory and motor neurons, we were able to identify the first links in the chain of biochemical events leading to short-term memory storage (figure 16–4).
That did not tell us how serotonin and cyclic AMP bring about the slow synaptic potential or how this synaptic potential relates to the enhanced release of glutamate, however. In 1980 I met Steven Siegelbaum in Paris, where I was giving a series of seminars at the College of France. Steve was a technically gifted young biophysicist who specialized in studying the properties of single ion channels. We hit it off extremely well and, as fate would have it, he had recently accepted a position in the pharmacology department at Columbia. We therefore decided to join forces upon his arrival in New York and explore the biophysical nature of the slow synaptic potential.
Steve discovered one of the targets of cyclic AMP and protein kinase A: a potassium ion channel in sensory neurons that responds to serotonin. We called this channel the S channel because it responds to serotonin and because it was discovered by Steve Siegelbaum. The channel is open when the neuron is at rest and contributes to its resting membrane potential. Steve found that the channel is present in the presynaptic terminals and that he could cause it to close either by applying serotonin (the first messenger) to the outside of the cell membrane or by applying cyclic AMP (the second messenger) or protein kinase A to the inside. Closing the potassium ion channel causes the slow synaptic potential that drew our attention to cyclic AMP in the first place.
Closing the channel also helps to enhance the release of glutamate. When the channel is open, it contributes, along with other potassium channels, to the resting membrane potential and to the outward movement of potassium during the descending stroke of the action potential. But when it is closed by serotonin, the ions move out of the cell less rapidly, slightly increasing the duration of the action potential by slowing the descending stroke. Steve showed that slowing the action potential allows more time for calcium to flow into the presynaptic terminals—and calcium, as Katz had shown in the squid’s giant synapse, is essential for the release of glutamate. In addition, cyclic AMP and protein kinase A act directly on the machinery that releases the synaptic vesicles, thus stimulating the release of glutamate even further.
These exciting results regarding cyclic AMP were soon complemented by important genetic studies of learning in fruit flies, a research favorite for over half a century. In 1907 Thomas Hunt Morgan at Columbia began to use the fruit fly,
Drosophila
, as a model organism for genetic studies because of its small size and short reproductive cycle (twelve days). This proved to be a happy choice because
Drosophila
has only four pairs of chromosomes (compared with twenty-three pairs in humans), making it a relatively easy animal to study genetically. It had long been obvious that many physical characteristics of animals—the shape of the body, eye color, and speed, among others—are inherited. If external physical characteristics can be inherited, can mental characteristics produced by the brain also be inherited? Do genes have a role in a mental process such as memory?
The first person to address this question with modern techniques was Seymour Benzer, at the California Institute of Technology. In 1967 he began a brilliant series of experiments in which he treated flies with chemicals designed to produce random mutations, or changes, in single genes. He then examined the effects of these mutations on learning and memory. To study memory in the fruit fly, Benzer’s students Chip Quinn and Yadin Dudai used a classical conditioning procedure. They placed the flies in a small chamber and exposed them to two odors in sequence. The flies were then given an electric shock in the presence of odor 1, teaching them to avoid that odor. Later, the flies were placed in another chamber with the sources of the two odors at opposite ends. The conditioned flies avoided the end containing odor 1 and streamed to the end containing odor 2.
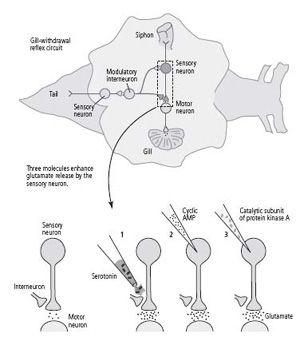
16–4 Molecules involved in short-term memory
. Applying serotonin to the terminal of a sensory neuron (
1
), injecting cyclic AMP into the neuron (
2
), and injecting the catalytic part of protein kinase A (
3
) all lead to increased release of the neurotransmitter glutamate. This suggests that each of these three substances participates in the pathway for short-term memory.