In Search of Memory: The Emergence of a New Science of Mind (26 page)
Read In Search of Memory: The Emergence of a New Science of Mind Online
Authors: Eric R. Kandel
Tags: #Psychology, #Cognitive Psychology & Cognition, #Cognitive Psychology
Third, we found that in all three forms of learning, the duration of short-term memory storage depends on the length of time a synapse is weakened or strengthened.
Fourth, we were beginning to understand that the strength of a given chemical synapse can be modified in two ways, depending on which of two neural circuits is activated by learning—a mediating circuit or a modulatory circuit. In
Aplysia
, the mediating circuit is made up of the sensory neurons that innervate the siphon, the interneurons, and the motor neurons that control the gill-withdrawal reflex. The modulatory circuit is made up of sensory neurons that innervate the tail in a completely different part of the body. When the neurons in a mediating circuit are activated, homosynaptic changes in strength occur. This is the case in habituation: the sensory and motor neurons that control the gill-withdrawal reflex fire repeatedly and in a certain pattern in direct response to the repeated sensory stimulus. Heterosynaptic changes in strength occur when the neurons in a modulatory, rather than the mediating, neural circuit are activated. This is the case with sensitization: the strong stimulus to the tail activates a modulatory circuit that controls the strength of synaptic transmission in the mediating neurons.
We later found that classical conditioning recruits both homosynaptic and heterosynaptic changes. Indeed, our studies of the relationship of sensitization to classical conditioning indicate that learning may be a matter of combining various elementary forms of synaptic plasticity into new and more complex forms, much as we use an alphabet to form words.
I now began to realize that the abundance of chemical over electrical synapses in the brains of animals may reflect a fundamental advantage of chemical over electrical transmission: the ability to mediate a variety of forms of learning and of memory storage. Viewed from this perspective, it became clear that the synapses between sensory neurons and motor neurons in the gill-withdrawal circuit—neurons that have evolved to participate in various types of learning—are much more easily changed than synapses that play no role in learning. Our studies showed dramatically that in circuits modified by learning, synapses can undergo large and enduring changes in strength after only a relatively small amount of training.

(Reprinted from
Behavioral Biology
of Aplysia, E. R. Kandel, W. H. Freeman and Company, 1979.)
One of the fundamental features of memory is that it is formed in stages. Short-term memory lasts minutes, while long-term memory lasts many days or even longer. Behavioral experiments suggest that short-term memory grades naturally into long-term memory and, moreover, that it does so through repetition. Practice does make perfect.
How does practice do it? How does training convert a short-term memory into a persistent, self-maintained long-term memory? Does the process take place at the same site—the connection between the sensory and motor cells—or is a new site required? We were now in a position to answer those questions.
In this period, science was again claiming my total concentration, to the exclusion of other activities. In my obsession with
Aplysia
, however, I found an unexpected ally in my daughter, Minouche. In 1970, at age five, as Minouche started to read, she stumbled on a picture of
Aplysia
in
The Larousse Encyclopedia of Animal Life
, a beautiful picture book that I kept in our living room. She simply loved the picture and would squeal,
“Aplysia! Aplysia!”
over and over again, pointing to the picture.
Two years later, at the age of seven, she wrote the following poem on the occasion of my forty-third birthday:
The Aplisa
by Minouche
An aplisa is like
a squishy snail.
In rain, in snow, in sleet,
in hail
.
When it is angry, it shoots
out ink
.
The ink is purple, it’s not
pink
.
An aplisa cannot live on
land
.
It doesn’t have feet so
it can’t stand
.
It has a very funny
mouth
.
And in winter it goes to the south
.
Minouche said it all, much better than I could!
I
had learned from my research in
Aplysia
that changes in behavior are accompanied by changes in the strength of the synapses between neurons that produce the behavior. But nothing in my research revealed how short-term memory is transformed into long-term memory. Indeed, nothing was known about the cellular mechanisms of long-term memory.
The basis for my early research in learning and memory was the learning paradigms used by behaviorists. The behaviorists focused primarily on how knowledge was acquired and stored in short-term memory. Long-term memory did not particularly interest them. The interest in long-term memory came from studies of human memory by the forerunners of cognitive psychologists.
IN 1885, A DECADE BEFORE EDWARD THORNDIKE BEGAN HIS
studies of learning in experimental animals at Columbia University, the German philosopher Hermann Ebbinghaus transformed the analysis of human memory from an introspective study into a laboratory science. Ebbinghaus was influenced by three scientists—the physiologist Ernst Weber, and the physicists Gustav Fechner and Hermann Helmholtz—who introduced rigorous methods to the study of perception. Helmholtz, for example, measured the speed at which a touch on the skin travels to the brain. It was generally thought at the time that conduction along nerves was immeasurably fast, comparable to the speed of light. But Helmholtz found it to be slow—about 90 feet per second. Moreover, the time it took a subject to react to the stimulus—the reaction time—was even slower! This caused Helmholtz to propose that a considerable amount of the brain’s processing of perceptual information is carried out unconsciously. He called this processing “unconscious inference” and proposed that it was based on evaluating and transforming the neural signal without conscious awareness of doing so. This processing, he argued, must result from signals being routed and processed at different sites during perception and voluntary movement.
Like Helmholtz, Ebbinghaus held the position that mental processes are biological in nature and can be understood in the same rigorous scientific terms as physics and chemistry. Perception, for example, can be studied empirically as long as the sensory stimuli used to elicit responses are objective and quantifiable. Ebbinghaus conceived the idea of studying memory by means of a similar experimental approach. The techniques he devised for measuring memory are still in use today.
In devising his experiments on how new information is put into memory, Ebbinghaus had to be certain that the people he studied were forming new associations, not relying for help on associations learned earlier. He hit upon the idea of having subjects learn nonsense words, each consisting of two consonants separated by a vowel (RAX, PAF, WUX, CAZ, and so on). Because each word is meaningless, it does not fit into the learner’s preestablished network of associations. Ebbinghaus made up about 2000 such words, wrote each on a separate slip of paper, shuffled the slips, and randomly drew slips to create lists that varied in length between 7 and 36 nonsense words. Faced with the arduous task of memorizing the lists, he went to Paris and rented a room in a garret overlooking the roofs of that beautiful city. There, he memorized each list in turn by reading it aloud at the rate of 50 words per minute. As Denise would say, “Only in Paris can one even think of doing such a boring experiment!”
From these self-inflicted experiments, Ebbinghaus generated two principles. First, he found that memory is graded—in other words, practice makes perfect. There was a linear relationship between the number of training repetitions on the first day and the amount of material retained on the following day. Long-term memory therefore appeared to be a simple extension of short-term memory. Second, despite the apparent similarity in mechanism between short- and long-term memory, Ebbinghaus noted that a list of six or seven items could be learned and retained in only one presentation, whereas a longer list required repeated presentations.
Next, he plotted a forgetting curve. He tested himself at various intervals after learning, using different lists for each interval, and determined the amount of time it took to relearn each list with the same degree of accuracy as in the first learning. He found that there were savings: relearning an old list took less time and fewer trials than the original learning. Most interesting of all, he found that forgetting had at least two phases: a rapid initial decline that was sharpest in the first hour after learning and then a much more gradual decline that continued for about a month.
Based on Ebbinghaus’s two phases of forgetting and on his own remarkable intuition, William James concluded in 1890 that memory must have at least two different processes: a short-term process, which he called “primary memory,” and a long-term process, which he called “secondary memory.” He referred to long-term memory as secondary because it involved recalling memory some time after a primary learning event.
IT GRADUALLY BECAME CLEAR TO THE PSYCHOLOGISTS WHO
followed Ebbinghaus and James that the next step in understanding long-term memory was to understand how it becomes firmly established, a process now called consolidation. For a memory to persist, the incoming information must be thoroughly and deeply processed. This is accomplished by attending to the information and associating it meaningfully and systematically with knowledge already well established in memory.
The first clue that newly stored information is made more stable for long-term storage came in 1900 from two German psychologists, Georg Müller and Alfons Pilzecker. Using Ebbinghaus’s techniques, they asked a group of volunteers to learn a list of nonsense words well enough to remember them twenty-four hours later, which the group did readily. They then asked a second group to learn the same list with the same number of repetitions, but they gave the second group an additional list of words to learn
immediately after
learning the first list. This second group of volunteers failed to remember the first list twenty-four hours later. In contrast, a third group of volunteers, who were given the second list
two hours after
having learned the first list, had little difficulty remembering the first list twenty-four hours later. This result suggested that in the hour after training, when the initial list had been placed into short-term memory and perhaps even into the early stages of long-term memory, memory was still sensitive to disruption. Presumably, a certain amount of time was required for long-term memory to become fixed, or consolidated. Once consolidated, after two or more hours, it was stable for some time and less easily disrupted.
The idea of memory consolidation is supported by two types of clinical observations. First, it has been known since the end of the nineteenth century that head injuries and concussion can lead to a memory loss called retrograde amnesia. A boxer who is hit on the head and sustains a brain concussion in round five of a match will usually remember going to the event, but everything after that will be blank. Undoubtedly, a number of events entered into his short-term memory just before the blow—the excitement of entering the ring, his opponent’s movements during the earlier four rounds, perhaps even the moving punch itself and the attempt to avoid it—but the blow to the brain occurred before any of those memory traces could be consolidated. The second clinical observation is that a similar retrograde amnesia often occurs following an epileptic convulsion. People with epilepsy cannot remember events immediately preceding a seizure, even though the seizure has no effect on their memory of earlier events. This suggests that in its early phases memory storage is dynamic and sensitive to disruption.
The first rigorous test of memory consolidation came in 1949, when the American psychologist C. P. Duncan applied electrical stimuli to the brain of animals during or immediately after training, resulting in convulsions that disrupted memory and caused retrograde amnesia. Producing seizures several hours after training had little or no effect on recall. Almost twenty years later, Louis Flexner at the University of Pennsylvania made the remarkable discovery that drugs that inhibit the synthesis of proteins in the brain disrupt long-term memory if given during and shortly after learning, but they do not disrupt short-term memory. This finding suggested that long-term memory storage requires the synthesis of new proteins. Together, the two sets of studies seemed to confirm the idea that memory storage takes place in at least two stages: a short-term memory lasting minutes is converted—by a process of consolidation that requires the synthesis of new protein—into stable, long-term memory lasting days, weeks, or even longer.
Variants of the two-stage model of memory were soon proposed. According to one view, short- and long-term memory take place at different anatomical sites. Alternatively, some psychologists argued that memory was present at one site and simply became progressively stronger with time. The question of whether short- and long-term memory require two separate sites or can be accommodated at one site is central to the analysis of learning, particularly to the analysis of memory on the cellular level. Clearly, it could not be resolved with behavioral analyses alone—cellular analysis was needed. Our studies of
Aplysia
had put us in a position to tackle the question of whether short- and long-term memory are the same or separate neural processes occurring at the same or different sites.
CAREW AND I HAD FOUND IN 1971 THAT, WITH REPEATED TRAINING
, habituation and sensitization—the simplest forms of learning—can be sustained for long periods. Thus they could serve as useful tests for differences between long-term and short-term memory. We eventually discovered that the cellular changes accompanying long-term sensitization in
Aplysia
were similar to changes underlying long-term memory in the mammalian brain: long-term memory required the synthesis of new protein.
We wanted to know whether simple forms of long-term memory use the same storage sites—the same group of neurons and the same set of synapses—as short-term memory. I knew from the work of Brenda Milner on H.M. that, in people, complex, explicit long-term memory—a memory lasting days to years—requires not only the cortex but also the hippocampus. But what about the simpler implicit memory? Carew, Castellucci, and I found that the same synaptic connections between sensory and motor neurons that are altered in short-term habituation and sensitization are also altered in long-term habituation and sensitization. Moreover, in both cases, the synaptic changes parallel the changes in behavior we observed: in long-term habituation, the synapse is depressed for a period of weeks, whereas in long-term sensitization, it is enhanced for weeks. This suggested that, in the simplest cases, the same site can store both short- and long-term memory and that it can do so for different forms of learning.
That left the question of mechanism. Are the mechanisms of short-term and long-term memory the same? If so, what is the nature of the process by which long-term memory is consolidated? Is protein synthesis needed for the long-term synaptic changes associated with long-term memory storage?
I had thought for some time that long-term memory might be consolidated by an anatomical change. Such a change might be one of the reasons that new protein is needed. I sensed that we would soon require an analysis of the structure of memory storage. In 1973 I succeeded in recruiting Craig Bailey, a talented and creative young cell biologist, to explore the structural changes that accompany the transition from short- to long-term memory.
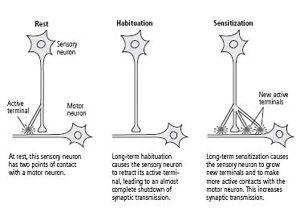
Bailey and his colleague, Mary Chen, and Carew and I found that long-term memory is not simply an extension of short-term memory: not only do the changes in synaptic strength last longer but, more amazingly, the actual number of synapses in the circuit changes. Specifically, in long-term habituation the number of presynaptic connections among sensory neurons and motor neurons decreases, whereas in long-term sensitization sensory neurons grow new connections that persist as long as the memory is retained (figure 15–1). There is in each case a parallel set of changes in the motor cell.
This anatomical change is expressed in several ways. Bailey and Chen found that a single sensory neuron has approximately 1300 presynaptic terminals with which it contacts about 25 different target cells—motor neurons, excitatory interneurons, and inhibitory interneurons. Of the 1300 presynaptic terminals, only about 40 percent have active synapses, and only these synapses have the machinery for releasing a neurotransmitter. The remaining terminals are dormant. In long-term sensitization, the number of synaptic terminals more than doubles (from 1300 to 2700), and the proportion of active synapses increases from 40 percent to 60 percent. In addition, there is an outgrowth from the motor neuron to receive some of the new connections. In time, as the memory fades and the enhanced response returns to normal, the number of presynaptic terminals drops from 2700 to about 1500, or slightly more than the initial number. This residual growth presumably is responsible for the fact, first discovered by Ebbinghaus, that an animal can learn a task more readily a second time. In long-term habituation, on the other hand, the number of presynaptic terminals drops from 1300 to about 850, and the number of active terminals diminishes from 500 to about 100—an almost complete shutdown of synaptic transmission (figure 15–1).