In Search of Memory: The Emergence of a New Science of Mind (24 page)
Read In Search of Memory: The Emergence of a New Science of Mind Online
Authors: Eric R. Kandel
Tags: #Psychology, #Cognitive Psychology & Cognition, #Cognitive Psychology
Alden’s and my review convinced David Cohen, a friendly rival who later became a colleague and vice president for arts and sciences at Columbia, of the value of simple systems. Because he was committed to vertebrates, Cohen turned to the pigeon, the favorite experimental animal of Skinner. But whereas Skinner ignored the brain, Cohen focused on brain-controlled changes in heart rate resulting from sensitization and classical conditioning.
Joseph LeDoux, who was also influenced by the review, modified Cohen’s protocol for classical conditioning and applied it to the rat, developing what has emerged as the best experimental system for studying the cellular mechanisms of learned fear in mammals. LeDoux focused on the amygdala, a structure that lies deep under the cerebral cortex and is specialized for the detection of fear. Years later, when it proved possible to produce genetically modified mice, I turned to the amygdala and, influenced by LeDoux’s work, extended the molecular biology of learned fear in
Aplysia
to learned fear in the mouse.
W
hen I arrived at NYU in December 1965 I knew the time had come to take a big step. In Tauc’s laboratory I had found that in response to different patterns of stimulation modeled on those produced by Pavlovian learning, a synapse can readily undergo long-lasting changes and that these affect the strength of the communication between two nerve cells in an isolated ganglion. But this was an artificial situation. I had no direct evidence that, in a behaving animal, actual learning leads to changes in the effectiveness of synapses. I needed to move beyond modeling learning in individual cells of an isolated ganglion to studying instances of learning and memory in the neural circuit of a behavior in the intact, behaving animal.
I therefore set two goals for the next several years. First, I would develop a detailed catalog of the behavioral repertoire of
Aplysia
and determine which behaviors could be modified by learning. Second, I would select for research one behavior capable of being modified by learning and use it to explore how learning occurs and how memories are stored in the neural circuitry of that behavior. I had this agenda in mind while I was still at Harvard, and I set about finding a postdoctoral fellow with a specific interest in learning in invertebrates to collaborate with me on this problem.
I had the good fortune to recruit Irving Kupfermann, a gifted and idiosyncratic behaviorist trained at the University of Chicago. He joined me in Boston a few months before I left Harvard and then moved with me to NYU. Irving was a typical University of Chicago intellectual. Tall and very thin, extremely bookish, and slightly eccentric, he wore thick glasses and was almost bald despite his youth. One of his students later described him as “a large brain at the end of a long thin rod.” Irving was allergic to rodents and cats, so he had worked on a small segmented land invertebrate creature, the pill bug, for his Ph.D. dissertation. He proved an extremely well informed and creative student of behavior who was very clever at designing experiments.
Together, we set about exploring the behavior of
Aplysia
, searching for one behavior that could be used to study learning. We became familiar with almost every feature of the animal’s feeding behavior, its daily pattern of locomotor activity (figure 13–1), inking, and egg laying. We were fascinated by its sexual behavior (figure 13–2), the most obvious and impressive social behavior in
Aplysia
. These snails are hermaphrodites; they can be both male and female, with different partners at different times or even simultaneously. By recognizing one another appropriately they can form impressive copulating chains in which each member serves as both male for the animal in front of it and female for the animal behind it in the chain.
As we analyzed and thought about these behaviors, we realized they were all too complex, some involving more than one ganglion of the snail’s nervous system. We needed to find a very simple behavior controlled by the cells of one ganglion. We therefore focused on the several behaviors controlled by the abdominal ganglion, the one I had studied in Paris and with which I was most familiar. The abdominal ganglion, which contains only two thousand nerve cells, controls heart rate, respiration, egg laying, inking, release of mucus, and withdrawal of the gill and siphon. In 1968 we settled on the simplest behavior: the gill-withdrawal reflex.
The gill is an external organ that
Aplysia
uses to breathe. It lies in a cavity of the body wall called the mantle cavity and is covered by a sheet of skin called the mantle shelf. The mantle shelf ends in the siphon, a fleshy spout that expels seawater and waste from the mantle cavity (figure 13–3A). Touching the siphon lightly produces a brisk defensive withdrawal of both the siphon and the gill into the mantle cavity (figure 13–3B). The purpose of the withdrawal reflex is clearly to protect the gill, a vital and delicate organ, from possible damage.
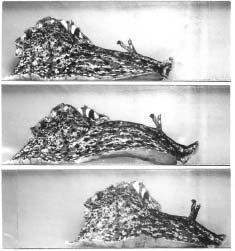
13–1 One full step
.
Aplysia
moves by lifting its head and releasing suction to raise the front of its foot, which it then extends for a distance equal to half its body length. The animal brings the front of its foot down, attaches it to a surface, then contracts its anterior end to take up the slack. (Courtesy of Paul Kandel.)
Irving and I found that even this very simple reflex can be modified by two forms of learning—habituation and sensitization—and each gave rise to a short-term memory that lasts for a few minutes. An initial light touch to the siphon produces brisk withdrawal of the gill. Repeated light touches produce habituation: the reflex progressively weakens as the animal learns to recognize that the stimulus is trivial. We produced sensitization by applying a strong shock to either the head or the tail. The animal recognized the strong stimulus as noxious and subsequently produced an exaggerated gill-withdrawal reflex in response to the same light touch to the siphon (figure 13–3C).
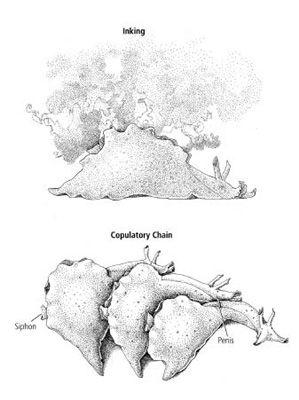
13–2 Simple and complex behaviors in
Aplysia
. Inking (above) is a relatively simple behavior, controlled by cells in a single ganglion (the abdominal ganglion) of the snail’s nervous system. Sexual behavior is far more complex and involves nerve cells in several ganglia.
Aplysia
are hermaphrodites, able to be both male and female, and often form copulatory chains such as the one shown (below). (Reprinted from
Cellular Basis of Behavior
, E. R. Kandel, W. H. Freeman and Company, 1976.)
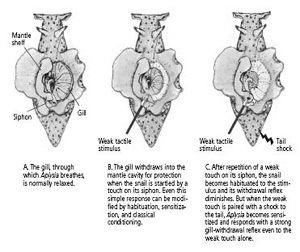
13–3
Aplysia
’s simplest behavior, the gill-withdrawal reflex.
In 1971 we were joined by Tom Carew, a gifted, energetic, and gregarious physiological psychologist from the University of California, Riverside, who opened up our study of long-term memory. Carew simply loved being in the neurobiology and behavior group at NYU. He became good friends with Jimmy Schwartz and Alden Spencer as well as with me. Like a dry sponge, Carew soaked up the culture of the group—not only the science, but also the shared interest in art, music, and scientific gossip. As Carew and I would say to each other, “When other people engage in this talk, it’s gossip; when we do it, it’s intellectual history.”
Carew and I found that long-term memory in
Aplysia
, as in people, requires repeated training interspersed with periods of rest. Practice makes perfect, even in snails. Thus, forty stimuli administered consecutively result in habituation of gill withdrawal that lasts only one day, but ten stimuli every day for four days produce habituation that lasts for weeks. Spacing the training with periods of rest enhances the ability of an
Aplysia
to establish long-term memory.
Kupfermann, Carew, and I had demonstrated that a simple reflex was amenable to two nonassociative forms of learning, each with short- and long-term memory. In 1983 we succeeded in reliably producing classical conditioning of the gill-withdrawal reflex. This was an important advance since it demonstrated that the reflex can also be modified by associative learning.
By 1985, after more than fifteen years of hard work, we had shown that a simple behavior in
Aplysia
could be modified by various forms of learning. This strengthened my hope that some forms of learning had been conserved throughout evolution and would be found even in a simple neural circuit of a very simple behavior. Moreover, I could now foresee the possibility of going beyond the questions of how learning occurs and how memory is stored in the central nervous system to the question of how different forms of learning and memory relate to each other at the cellular level. Specifically, how is short-term memory converted to long-term memory in the brain?
BEHAVIORAL STUDIES OF THE GILL-WITHDRAWAL REFLEX WERE
not the sole focus of our work during this time. Indeed, they formed the groundwork for our second and principal interest—devising experiments to explore what happens in the brain of an animal as it learns. Thus, once we had decided to focus our study of learning on the gill-withdrawal reflex in
Aplysia
, we needed to map the neural circuitry of the reflex to learn how the abdominal ganglion brings it about.
Working out the neural circuitry posed its own conceptual challenge. How precise and specialized are the connections among the cells of a neural circuit? In the early 1960s some followers of Karl Lashley argued that the properties of the different neurons in the cerebral cortex are so similar that they are to all intents and purposes identical, and their interconnections random and of roughly equal value.
Other scientists, particularly students of the invertebrate nervous system, championed the idea that many, perhaps all, neurons are unique. This idea was first proposed in 1908 by the German biologist Richard Goldschmidt. Goldschmidt studied a ganglion of the nematode worm
Ascaris
, a primitive intestinal parasite. He found that every animal of the species has the same number of cells in exactly the same position in that ganglion. In a now famous lecture to the German Zoological Association that year, he noted, “the almost startling constancy of the elements of the nervous system: There are 162 ganglion cells in the center, never one more nor less.”
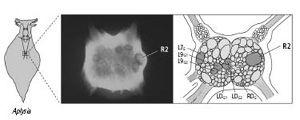
13–4 Identifying specific neurons in the abdominal ganglion of
Aplysia
. Cell R2 can be clearly seen in a photomicrogaph (left) of
Aplysia
’s abdominal ganglion. It measures 1 millimeter in diameter. A drawing (right) shows the position of cell R2 and the six motor neurons controlling movement of the gill. Once individual neurons had been identified, it was possible to map their connections.
Angelique Arvanitaki-Chalazonitis was aware of Goldschmidt’s analysis of the worm, and in the 1950s she explored the abdominal ganglion of
Aplysia
in search of identifiable cells. She found that several cells are unique and identifiable in every individual animal on the basis of their location, pigmentation, and size. One such cell was R2, the cell I had focused on in my studies of learning with Ladislav Tauc. First at Harvard and later at NYU, I followed up on this lead, and by 1967 I had found, as had Goldschmidt and Arvanitaki-Chalazonitis earlier, that I could easily identify most of the prominent cells in the ganglion (figure 13–4).