In Search of Memory: The Emergence of a New Science of Mind (33 page)
Read In Search of Memory: The Emergence of a New Science of Mind Online
Authors: Eric R. Kandel
Tags: #Psychology, #Cognitive Psychology & Cognition, #Cognitive Psychology
How the cell targets proteins to specific synapses was a question ideally suited for Kelsey Martin, an extremely gifted cell biologist who had obtained a combined M.D.-Ph.D. at Yale. After graduating from Harvard College, she and her husband joined the Peace Corps and worked in Africa. By the time they came to Columbia, they already had a son, Ben. While she was in our laboratory, they had a daughter, Maya. Kelsey proved a special presence in the laboratory, not only doing first-class science with extraordinary skill, but also lifting all of our spirits by turning our little conference-lunchroom into a joyful kindergarten for gifted children from 4:00 to 6:00
P.M.
The tracking of protein kinase A to the nucleus and the discovery in the nucleus of the CREB regulators had led us along a molecular pathway from the synapse to the nucleus. Now we had to begin the reverse journey. Kelsey and I needed to explore, in a single sensory cell, how a stimulated synapse that is undergoing long-term structural changes differs from an unstimulated synapse. We did this by developing an elegant new cell culture system.
We grew a single sensory neuron with a branched axon that formed synaptic connections with two separate motor neurons. We simulated behavioral training as before by applying pulses of serotonin, but now we could apply them selectively to one or the other set of synaptic connections. A single pulse of serotonin applied to one set of synapses produced short-term facilitation in those synapses only, as expected. Yet five pulses of serotonin applied to one set of synapses produced long-lasting facilitation and growth of new synaptic terminals only at the stimulated synapses. This result was surprising because long-term facilitation and growth require the activation of genes by CREB, an action that takes place in the nucleus of the cell and should theoretically affect all of the cell’s synapses. When Kelsey blocked the action of CREB in the nucleus of the cell, it suppressed both facilitation and growth at the stimulated synapse (figure 19–2).
This finding gave us tremendous insight into the computational power of the brain. It illustrates that even though a neuron may make one thousand or more synaptic connections with different target cells, the individual synapses can be modified independently, in long-term as well as short-term memory. The synapses’ independence of long-term action gives the neuron extraordinary computational flexibility.
How does this extraordinary selectivity come about? We considered two possibilities: Do the neurons ship messenger RNA and proteins only to synapses marked for long-term memory storage? Or are messenger RNA and proteins shipped to all of the neuron’s synapses and only the marked synapses are able to utilize them for growth? We first began by testing the second hypothesis because it was easy to explore.
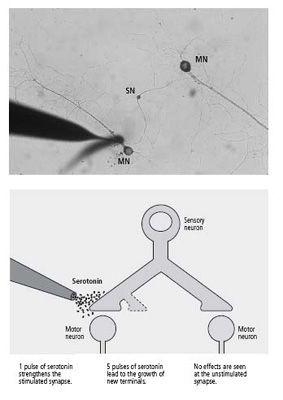
19–2 Setup for studying the role of serotonin in synaptic change
. A sensory neuron (SN in photo, above) with a branched axon forms synapses with two motor neurons (MN). Serotonin is applied to just one of the synapses. Only that synapse undergoes short- and long-term change. (Image courtesy of Kelsey Martin.)
What makes this “marked for growth” process possible? Kelsey found that two things must occur at the marked synapse. The first is simply the activation of protein kinase A. If protein kinase A is not activated at the synapse, no facilitation occurs at all. The second is the activation of machinery that regulates
local
protein synthesis. This was a very surprising finding, and it made new sense out of a fascinating area of nerve cell biology that had not been fully appreciated and therefore had been largely ignored. In the early 1980s Oswald Steward, now at the University of California, Irvine, had discovered that even though the vast majority of protein synthesis takes place in the cell body of the neuron, some also occurs locally, at the synapses themselves.
Our findings now indicated that one function of local protein synthesis is to sustain the long-term strengthening of the synaptic connection. When we inhibited local protein synthesis at a synapse, the process of long-term facilitation began and new terminals grew, making use of the proteins sent to the synapse from the cell body. That new growth could not be sustained, however, and after one day it regressed. Thus the proteins synthesized in the cell body and shipped to the terminals are sufficient to initiate synaptic growth, but to sustain that growth, proteins synthesized locally are necessary (figure 19–3).
These results opened up a new window on long-term memory. They suggested that two independent mechanisms are at work. One process initiates long-term synaptic facilitation by sending protein kinase A to the nucleus to activate CREB, thereby turning on the effector genes that encode the proteins needed for the growth of new synaptic connections. The other process perpetuates memory storage by maintaining the newly grown synaptic terminals, a mechanism that requires local protein synthesis. Thus we realized that there are separate processes for initiation and for maintenance. How does that second mechanism work?
IT WAS AT THIS POINT IN 1999 THAT KAUSIK SI, A REMARKABLY
original and effective scientist, joined the laboratory. Kausik came from a small town in India, where his father taught in the local high school. When Kausik’s father realized that Kausik was interested in biology, he asked a colleague, the local biology teacher, to take the boy under his wing. This biology teacher taught Kausik a great deal and engendered his interest in genetic mechanisms. The teacher also encouraged Kausik to seek graduate training in biology in the United States, which ultimately led to his taking his postdoctoral training with me at Columbia.
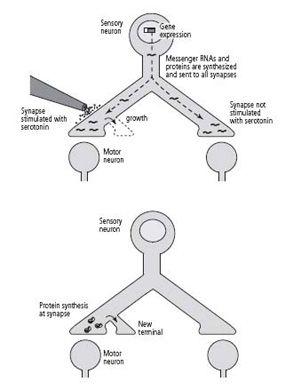
19–3 Two mechanisms of long-term change
. New proteins are sent to all of the synapses (above), but only synapses stimulated with serotonin use them to initiate the growth of new axon terminals. Proteins synthesized locally (below) are needed to sustain the growth initiated by gene expression.
Kausik had done his Ph.D. research on protein synthesis in yeast, and after arriving at Columbia, he began to think about the problem of local protein synthesis in
Aplysia
. We knew that messenger RNA molecules are synthesized in the nucleus and translated into protein at specific synapses. So the question was this: Is the messenger RNA sent to the terminals in an active state? Or is it sent in a dormant state, like Sleeping Beauty waiting to be kissed at the marked synapses by some molecular Prince Charming?
Kausik favored the Sleeping Beauty hypothesis. He argued that the dormant messenger RNA molecules become activated only if they reach an appropriately marked synapse and encounter a particular signal. He pointed out that an interesting example of this sort of regulation occurs in the development of the frog. As the frog’s egg becomes fertilized and matures, dormant messenger RNA molecules are awakened and activated by a novel protein that regulates local protein synthesis. This protein is known as CPEB (cytoplasmic polyadenylation element-binding protein).
As we journeyed deeper into the maze of molecular processes underlying memory, Kausik found that a novel form of CPEB in
Aplysia
was indeed the Prince Charming we had sought. The molecule is present only in the nervous system, is located at all the synapses of a neuron, is activated by serotonin, and is required at the activated synapses to maintain protein synthesis and the growth of new synaptic terminals. But Kausik’s finding advanced the question just one step further. Most proteins are degraded and destroyed in a period of hours. What maintains growth over longer periods of time? What, in principle, has sustained my memory of Mitzi for a lifetime?
As Kausik looked carefully at the amino acid sequence of the novel CPEB, he noticed something very peculiar. One end of the protein had all the characteristics of a prion.
Prions are probably the weirdest proteins known to modern biology. They were first discovered by Stanley Prusiner of the University of California, San Francisco as the causal agents of several mysterious neurodegenerative diseases, such as mad cow disease (bovine spongiform encephalopathy) in cattle and Creutzfeldt-Jakob disease in people (this is the disease that tragically killed Irving Kupfermann in 2002, at the prime of his scientific career). Prions differ from other proteins in that they can fold into two functionally distinct shapes, or conformations; one of these is dominant, the other recessive. The genes that encode prions give rise to the recessive form, but the recessive form can be converted to the dominant form, either by chance alone, as may have happened with Irving, or by eating food that contains an active form of the protein. In the dominant form, prions can be lethal to other cells. The second way in which prions differ from other proteins is that the dominant form is self-perpetuating; it causes the recessive conformation to change its shape and thereby become dominant and self-perpetuating as well (figure 19–4).
I remember it was a beautiful New York afternoon in the spring of 2001, the bright sunlight rippling off the Hudson River outside my office windows, when Kausik walked into my office and asked, “What would you say if I told you that the CPEB has prion-like properties?”
A wild idea! But if true, it could explain how long-term memory is maintained in synapses indefinitely, despite constant protein degradation and turnover. Clearly, a self-perpetuating molecule could remain at a synapse indefinitely, regulating the local protein synthesis needed to maintain newly grown synaptic terminals.
In my late-night thoughts about long-term memory, I had once briefly entertained the idea that prions might somehow be involved in long-term memory storage. Moreover, I was familiar with Prusiner’s groundbreaking work on prions and prion diseases, work for which he would receive the 1997 Nobel Prize in Medicine or Physiology. Thus, while I had never anticipated that the novel form of CPEB might be a prion, I was immediately enthusiastic about Kausik’s ideas.
Prions were a major field of study in yeast, but no one had identified a normal function of these proteins until Kausik’s discovery of the novel form of CPEB in neurons. Thus his discovery not only offered deep new insights into learning and memory, it broke new ground in biology. We soon found that in the sensory neurons of the gill-withdrawal reflex, the conversion of CPEB from the inactive, non-propagating form to the active, propagating form is controlled by serotonin, the transmitter that is required for converting short- to long-term memory (figure 19–4). In its self-perpetuating form, CPEB maintains local protein synthesis. Moreover, the self-perpetuating state is not easily reversed.
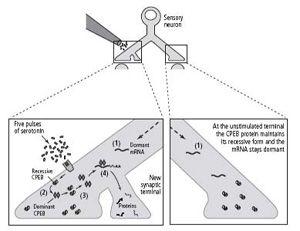
19–4 Long-term memory and the prion-like CPEB protein.
As a result of a prior stimulus, the sensory cell’s nucleus has sent dormant messenger RNA (mRNA) to all axon terminals (
1
). Five pulses of serotonin at one terminal convert a prion-like protein (CPEB) that is present at all synapses into a dominant, self-perpetuating form (
2
). Dominant CPEB can convert recessive CPEBs to the dominant form (
3
). Dominant CPEB activates dormant messenger RNA (
4
). The activated messenger RNA regulates protein synthesis at the new synaptic terminal, stabilizes the synapse, and perpetuates the memory.