Power, Sex, Suicide: Mitochondria and the Meaning of Life (17 page)
Read Power, Sex, Suicide: Mitochondria and the Meaning of Life Online
Authors: Nick Lane
Tags: #Science, #General
It works like this. Recall from the previous chapter that the complexes of the respiratory chain are plugged through the membrane. The hydrogen atoms that enter the respiratory chain are split into protons and electrons. The electrons pass down the chain like the current in a wire, via a succession of redox reactions (
Figure 7
). The energy released, said Mitchell, doesn’t form a high-energy chemical intermediate at all; the squiggle is elusive because it does not exist. Rather, the energy released by electron flow is used to pump protons across the membrane. Three of the four respiratory complexes use the energy released by electron flow to thrust protons across the membrane. The membrane is otherwise impermeable to protons, so any backflow is restricted, and a reservoir develops. Protons carry a positive charge, which means that the proton gradient has both an electrical and a concentration component. The electrical component generates a potential difference across the membrane, while the concentration component generates a difference in pH, or acidity (acidity is defined as proton concentration) with the outside more acid than the inside. The combination of pH and potential difference across the membrane constitutes what Mitchell called the ‘proton-motive force.’ It is this force that drives the synthesis of ATP. Because ATP is synthesized by the ATPase, Mitchell predicted that the ATPase would need to be powered by the proton-motive force—a current of protons flowing down the proton gradient from the pent-up reservoir; what Mitchell liked to call proton electricity, or proticity.
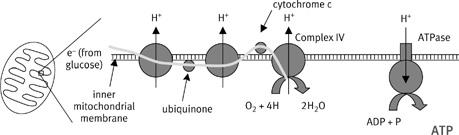
7
Simplified representation of the respiratory chain, as in
Figure 5
, but now showing the nature of the intermediate—the proton. Electrons (e
–
) pass down the chain from complex I to complex IV, and the energy released at each step is coupled to the expulsion of a proton across the membrane. This leads to a difference in proton concentration across the membrane, which can be measured as a difference in acidity (pH, or acidity, is defined as the proton concentration) and as an electrical potential difference, as protons carry a single positive charge. The reservoir of protons acts as a reservoir of potential energy, just as a reservoir of water on top of a hill acts as a reservoir of potential energy that can be used to generate hydroelectric power. Likewise, the flow of protons down the concentration gradient can be used to power mechanical tasks, in this case ATP synthesis. The flow of protons through the ATPase is called the ‘proton-motive force’ and it turns the tiny molecular motor of the ATPase, to generate ATP from ADP and phosphate.
Mitchell’s ideas were ignored, regarded with hostility, dismissed as mildly insane, or claimed as derivative. Racker later wrote: ‘Given the general attitude of the establishment, these formulations sounded like the pronouncements of a court jester or of a prophet of doom.’ The theory was couched in the strange, almost mystical, terminology of electrochemists, and made use of concepts that were unfamiliar to most enzymologists at the time. Only Racker and Bill Slater in Amsterdam (another protégé of Keilin) took it seriously at first, albeit with open-minded scepticism; and Slater soon lost his patience.
Mitchell, who was by turns brilliant, argumentative, irascible, and grandiloquent,
exacerbated the situation. He could infuriate his opponents. In an argument with Mitchell, Slater was seen hopping around on one foot in rage, illuminating the expression ‘hopping mad.’ These arguments took their toll on Mitchell too, who was forced to resign from Edinburgh University with stomach ulcers. In a two-year interim out of science, he restored the decaying eighteenth-century manor, Glynn House, near Bodmin in Cornwall, as a family home and private research institute. He returned to the research front in 1965, having girded up his loins for the battles to come. And come they did. The raging disputes of the next two decades were dubbed the ‘ox phos’ wars (from oxidative phosphorylation, the mechanism of ATP production in respiration).
Mitchell’s hypothesis neatly solved the nagging difficulties that dogged the older theories. It explained why a membrane was necessary, and why it had to be intact—a leaky membrane would allow a drizzle of protons back through, and so dissipate the proton-motive force as heat. A porous dam is no use to anyone.
It also explained how the mysterious uncoupling agents worked. Recall that ‘uncoupling’ refers to the loss of correspondence between glucose oxidation and ATP production, like a bike that loses its chain—the energy put into peddling is no longer connected to a useful function. Uncoupling agents all disconnect the energy input from the output, but otherwise seemed to have little else in common. Mitchell showed that they did have something in common—they are weak acids, which dissolve in the lipids of the membrane. Because weak acids can either bind or release protons, according to the acidity of their surroundings, they can shuttle protons across the membrane. In alkaline or weakly acidic conditions they lose a proton and gain a negative charge. Drawn by the electric charge they cross to the positive, acidic side of the membrane. Then, being a weak acid in strongly acidic conditions, they pick up a proton again. This neutralizes the electrical charge, so they become subject to the concentration gradient again. The weak acid traverses the membrane to the less acidic side, whereupon it loses the proton and once more becomes subject to the electrical tug. This kind of cycling can only happen if the uncoupling agent dissolves in the membrane regardless of whether it has bound a proton or not; and it was this subtle requirement that confounded earlier attempts at an explanation. (Some weak acids are soluble in lipids, but only when they have bound to a proton or vice versa; when they have released their proton they are no longer soluble in lipids, and so can’t cross back over the membrane; they therefore can’t uncouple respiration.)
Even more fundamentally, the chemiosmotic hypothesis explained the voodoo ‘action at a distance’ that seemed to beg a high-energy intermediate, the elusive squiggle. Protons pumped across a membrane in one spot generate a force that acts equally anywhere on the surface of the membrane, just as the pressure of water behind a dam depends on the overall volume of water, not on the location of the pump. So protons are pumped over the membrane in one place, but can return through an ATPase anywhere else in the membrane with a force that depends on the overall proton pressure. In other words, there was no
chemical
intermediate, but the proton-motive force itself acted as an intermediate—the energy released by respiration was conserved as the reservoir of protons. This also explained how a non-integer number of electrons could generate ATP—although a fixed number of protons are pumped across the membrane for every electron transported, some of the protons leak back through the dam, whereas others are tapped off for other purposes: they are not used to power the ATPase (we’ll return to this in the next section).
Perhaps most importantly, the chemiosmotic theory made a number of explicit predictions, which could be tested. Over the following decade, Mitchell, working in the refurbished Glynn House with his life-long research colleague Jennifer Moyle, and others, proved that mitochondria do indeed generate a pH gradient as well as an electrical charge (of about 150 millivolts) across the inner membrane. This voltage might not sound like a lot (it’s only about a tenth of that available from a torch battery) but we need to think of it in molecular terms. The membrane is barely 5 nm (10
–9
m) thick, so the voltage experienced from one side to the other is in the order of 30 million volts per metre—a similar voltage to a bolt of lightning, and a thousand times the capacity of normal household wiring. Mitchell and Moyle went on to show that a sudden rise in oxygen levels elicited a transient rise in the number of protons pumped over the membrane; they showed that respiratory ‘uncouplers’ really did work by shuttling protons back across the membrane; and they showed that the proton-motive force did indeed power the ATPase. They also demonstrated that proton pumping is coupled to the passage of electrons down the respiratory chain, and slows or even stops if any raw materials (hydrogen atoms, oxygen, ADP, or phosphate) run short.
By then, Mitchell and Moyle were not the only experimentalists working on chemiosmotics. Racker himself helped to convince the field by showing that if the respiratory complexes were isolated and then added to artificial lipid vesicles, they could still produce a proton gradient. But perhaps the one experiment that did more than any other to convince researchers, or botanists at least, of the veracity of the theory was carried out by André Jagendorf and Ernest Uribe at Cornell University in 1966. Jagendorf’s initial reaction to the chemiosmotic hypothesis had been hostile. He wrote: ‘I had heard Peter
Mitchell talk about chemiosmosis at a bioenergetics meeting in Sweden. His words went into one of my ears and out the other, leaving me feeling annoyed they had allowed such a ridiculous and incomprehensible speaker in.’ But his own experiments convinced him otherwise.
Working with chloroplast membranes, Jagendorf and Uribe suspended the membranes in acid, at pH 4, and gave the acid time to equilibrate across the membrane. Then they injected an alkali, at pH 8, into the preparation, creating a pH difference of 4 units across the membrane. They found that large amounts of ATP were created by this process, without the need for light or any other energy source: ATP synthesis was powered by the proton difference alone. Notice that I’m talking about
photosynthetic
membranes here. A striking feature of Mitchell’s theory is that it reconciles quite distinct modes of energy production that seem to be unrelated, like photosynthesis and respiration—both produce ATP via a proton-motive force across a membrane.
By the mid 1970s, most of the field had come round to Mitchell’s point of view—Mitchell even maintained a chart showing the dates when his rivals ‘converted’, to their fury—even though many molecular details still needed to be worked out, and remained controversial. Mitchell was sole recipient of the Nobel Prize for chemistry in 1978, another source of acrimony, although I believe his conceptual leap justified it. He had been through a personally traumatic decade, fighting poor health as well as a hostile bioenergetic establishment, but lived to see the conversion of his fiercest critics. In thanking them for their intellectual generosity in his Nobel lecture, Mitchell quoted the great physicist Max Planck—‘a new scientific idea does not triumph by convincing its opponents, but rather because its opponents eventually die.’ To have falsified this pessimistic dictum, said Mitchell, was a ‘singularly happy achievement.’
Since 1978, researchers have whittled away at the detailed mechanisms of electron transport, proton pumping, and ATP formation. The crowning glory was John Walker’s determination of the structure of the ATPase in atomic detail, for which he shared the Nobel Prize for chemistry in 1997 with Paul Boyer, who had suggested the basic mechanism many years earlier. (This was broadly similar in principle, but differed in detail, from the mechanism favoured by Mitchell.) The ATPase is a marvellous example of nature’s nano-technology: it works as a rotary motor, and as such is the smallest known machine, constructed from tiny moving protein parts. It has two main components, a drive shaft, which is plugged straight through the membrane from one side to the other, and a rotating head, which is attached to the drive shaft, resembling a mushroom head when seen down the electron microscope. The pressure of the proton reservoir on the outside of the membrane forces protons through the drive shaft to rotate the head; for each three protons that pass through the drive shaft, the head cranks around by 120°, so three cranks complete
a turn. There are three binding sites on the head, and these are where the ATP is assembled. Each time the head rotates, the tensions exerted force chemical bonds to form or break. The first site binds ADP; the next crank of the head attaches the phosphate onto the ADP to form ATP; and the third releases the ATP. In humans, a complete turn of the head requires 9 protons and releases 3 molecules of ATP. Just to complicate matters, in other species, the ATPase often requires different numbers of protons to rotate the head.
The ATPase is freely reversible. Under some circumstances it can go into reverse, whereupon it splits ATP, and uses the energy released to pump protons
up
the drive shaft, back across the membrane against the pressure of the reservoir. In fact the very name ATPase (rather than ATP synthase) signifies this action, which was discovered first. This bizarre trait hides a deep secret of life, and we’ll return to it in a moment.
In a broad sense, respiration generates energy using proton pumps. The energy released by redox reactions is used to pump protons across a membrane. The proton difference across the membrane corresponds to an electric charge of about 150 mV. This is the proton-motive force, which drives the ATPase motor to generate ATP, the universal energy currency of life.