Power, Sex, Suicide: Mitochondria and the Meaning of Life (20 page)
Read Power, Sex, Suicide: Mitochondria and the Meaning of Life Online
Authors: Nick Lane
Tags: #Science, #General
8
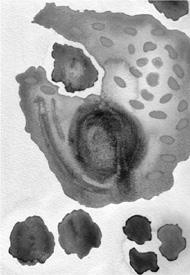
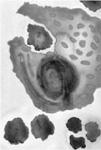
Primordial cells with iron-sulphur membranes.
(a) Electron micrograph of a thin section of iron-sulphur mineral (pyrites) from Tynagh, Ireland, 360 million years old. (b) Electron micrograph of structures formed in a laboratory by injecting sodium sulphide (NaS) solution, representing hydrothermal fluid, into iron chloride (FeCl
2
) solution, representing the iron-rich early oceans.
This is not just speculation. Russell and his long-term collaborator, Alan Hall, have simulated it in the laboratory. By injecting sodium sulphide solution (representing the hydrothermal fluid oozing up from the bowels of the earth) into iron chloride solution (representing the early oceans) Russell and Hall produced a host of tiny, microscopic bubbles, bounded by iron-sulphide membranes (
Figure 8
). These bubbles have two remarkable traits, which make me believe that Russell and Hall are thinking along the right lines. First, the cells are naturally chemiosmotic, with the outside more acidic than the inside. This situation is similar to the Jagendorf-Uribe experiment, in which a pH difference across the membranes was enough to generate ATP. Because Russell’s cells come with a natural pH gradient, all that the cells need to do to generate ATP is to plug an ATPase through the membrane, surely orders of magnitude easier to evolve than an entire functional fermentation pathway! If the first step on the way to the origin of life required little more than an ATPase, then Racker’s prescient description of the ATPase as the ‘elementary particle of life’ may have had more substance than even he could have known.
Second, the iron-sulphur crystals in the bubbly membranes conduct electrons (as indeed do the iron-sulphur proteins that still exist in mitochondrial membranes today). The reduced fluids that well up from the mantle are rich in electrons, whereas the relatively oxidized oceans are poor in electrons, setting up a potential difference across the membrane of several hundred millivolts—quite similar to the voltage across bacterial membranes today. This voltage stimulates the flow of electrons from one compartment to the other through the membrane. What’s more, the flow of negatively charged electrons draws positively charged protons from inside, giving rise to a rudimentary proton pumping mechanism.
Not only do the iron-sulphur cells provide a continuous supply of energy, but they also act as miniature electrochemical reactors, catalysing fundamental biochemical reactions, and concentrating the reaction products. The basic building blocks of life, including RNA, ADP, simple amino acids, small peptides, and so on, could all have been formed by virtue of the catalytic properties of the iron-sulphur minerals, and perhaps sedimental clays, in the reactions described by Gunter Wächtershäuser, but with two great advantages—they are concentrated by the membranes (preventing them from diffusing away into the oceans), and they are powered by a natural source of energy, the proton gradient.
Does all this sound improbable? In the previous chapter, I suggested that the origin of life was not as improbable as the evolution of the eukaryotes. Think about what is happening here. Such conditions could not have been rare on the early earth. Volcanic activity has been estimated to be fifteenfold greater than today. The crust was thinner, the oceans shallower, and the tectonic plates were only just forming. Volcanic seepage sites must have existed across much of the surface of the earth, to say nothing of more violent volcanic activity. The formation of many millions of tiny cells, bounded by iron-sulphur membranes, requires no more than a difference in redox state and acidity between the oceans and the volcanic fluids emanating from deep in the crust—a difference that certainly existed.
The early earth, as envisaged by Russell, is a giant electrochemical cell, which depends on the power of the sun to oxidize the oceans. UV rays split water and oxidize iron. Hydrogen, released from water, is so light that it is not retained by gravity, and evaporates off into space. The ocean becomes gradually oxidized, relative to the more reduced conditions in the mantle. According to the basic rules of chemistry, the mixing zone inevitably forms natural cells, replete with their own chemiosmotic and redox gradients. Mixing would have been assisted by the high tidal range, drawn the tug of the newly formed moon, which was closer to the earth then than today. We can be almost certain that such cells would really have formed, perhaps on a massive scale. And of course we can see their remains in the geology of places like Tynagh. There is a long way to go from here to make even a bacterium, but these conditions are a good first step.
Not only would the requisite conditions have been probable, but they would have been stable and continuous. They depend only on the power of the sun, without requiring problematic inventions like photosynthesis or fermentation. The sun is only needed to oxidize the oceans, as we know it must. Of all the forms of energy mooted by astrobiologists—meteorite impacts, volcanic heat, lightning—the power of the sun has often been curiously overlooked by scientists, if not by prehistoric mythologies. As the distinguished microbiologist Franklin Harold put it in his classic text,
The Vital Force
(the title of which I honour in the title of this Part): ‘One cannot help but suspect that the great stream of energy that passes across the earth plays a larger role in biology than our current philosophy knows: that perhaps the flood of power not only permitted life to evolve, but called it into being.’
For hundreds of millions of years, the sun provided the constant source of energy needed to pay the debt to the second law of thermodynamics. It created chemical disequilibria, and promoted the formation of naturally chemiosmotic cells. The primordial conditions are still faithfully replicated in the fundamental properties of all cells today. Both organic and inorganic cells are bounded
by a membrane, which physically contains the cell’s organic constituents, preventing them from diffusing away into the oceans. In both organic and inorganic cells, biochemical reactions are catalysed by minerals (today embedded as the prosthetic groups of enzymes). In both cases, the membrane is the barrier as well as carrier of energy. In both cases, energy is captured by a chemiosmotic gradient, with a positive charge and acidic conditions on the outside, and relatively negative, alkaline conditions on the inside. In both cases, redox reactions, electron transport and proton pumping regenerate the gradient. When the bacteria and archaea finally emerged from their nursery, to venture into the open oceans, they took with them an unmistakable seal of their origin. They parade it still today.
But this imprint, echoing the origin of life itself, was also life’s primary limitation. Why, we may ask, did bacteria never evolve beyond bacteria? Why did four billion years of bacterial evolution never succeed in producing a truly multicellular, intelligent bacterium? More specifically, why did the evolution of the eukaryotes require a union between an archaeon and a bacterium, rather than just the gradual accrual of complexity by a favoured line of bacteria or archaea? In
Part 3
, we’ll see that the answer to this long-standing riddle, and an explanation for the marvellous flowering of the eukaryotic line into plants and animals, lies in the fundamental nature of energy production by chemiosmotics across a bounding membrane.
3
Insider Deal
The Foundations of Complexity
Bacteria ruled supreme on Earth for two billion years. They evolved almost unlimited biochemical versatility but never discovered the secrets of greater size or morphological complexity. Life on other planets may get stuck in the same rut. On Earth, large size and complexity only became possible once energy generation had been internalized in mitochondria. But why did bacteria never internalize their own energy generation? The answer lies in the tenacious survival of mitochondrial DNA, a two-billion-year-old paradox.
A large cell with things inside—in eukaryotes, energy generation is internalized in mitochondria
Here is a list of words to make an evolutionary biologist spill their beer: purpose, teleology, ramp of ascending complexity, non-Darwinian. All these terms are associated with a religious view of evolution—the sense that life was ‘programmed’ to evolve, to become more complex, to give rise to humanity on a smooth curve from the lowest animals to the angels, each approaching closer to God—the great ‘chain of being’. Such a view is popular not just with religious theorists, but nowadays with astrobiologists too. The idea that the laws of physics virtually summon life forth in the universe that we see around us is a comforting one, and evokes the idea that even human sentience may be an inevitable outcome of the workings of physics. I disagreed in
Part 1
, and we will consider the theme further in
Part 3
by looking at the origin of biological complexity.
In
Part 1
, we observed that all complex multicellular organisms on earth are composed of eukaryotic cells; in contrast, bacteria have remained resolutely bacterial for the best part of four billion years. There is a chasm between bacterial and eukaryotic cells, and life elsewhere in the universe might well get stuck in the bacterial rut. We have seen that the eukaryotic cell was first formed in an unusual union between a bacterium and an archaeon. The question we’ll look into now is the ‘seeding’ of complexity in eukaryotes: what exactly is it about the eukaryotic cell that seems to encourage the evolution of complexity? However misleading the impression may be, surveying the grand canvas of evolution
after
the appearance of the eukaryotic cell
does
engender a sense of purpose. The idea of a great chain of being, striving to approach closer to God, is not accidental, even if it is wrong. In
Part 3
, we’ll see that the seeds of complexity were sown by mitochondria, for once mitochondria existed, life was almost bound to become more complex. The drive towards greater complexity came from within, not from on high.
In his celebrated book
Chance and Necessity
, the committed atheist and Nobel Prize-winning molecular biologist Jacques Monod tackled the theme of purpose. Plainly, he said, it is pointless to discuss the heart without mentioning that it is a pump, whose function is to pump blood around the body. But that is to ascribe purpose. Worse, if we were to say that the heart evolved
to
pump blood, we would be committing the ultimate sin of teleology—the assignment of a forward-looking purpose, a predetermined end-point to an evolutionary trajectory. But the heart could hardly have evolved ‘for’ anything else; if it didn’t evolve
to
pump blood, then it is truly a miracle that it happened to become so
fine a pump. Monod’s point was that biology is full of purpose and apparent trajectories, and it is perverse to pretend they don’t exist; rather, we must explain them. The question we must answer is this: how does the operation of blind chance, a random mechanism without foresight, bring about the exquisitely refined and purposeful biological machines that we see all around us?
Darwin’s answer, of course, was natural selection. Blind chance serves only to generate random variation within a population. Selection is not blind, or at least not random: it selects for the overall fitness of an organism in its particular environment—the survival of the fittest. The survivors pass on their successful genetic constitution to their offspring. Thus any changes that improve the function of the heart at pumping blood will be passed on, while any that undermine it will be eliminated by selection. In each generation (in the wild) only a few per cent might survive to reproduce, and they will tend to be the luckiest or best adapted. Over many generations luck no doubt balances out, so natural selection tends to select the best adapted of the best adapted, inevitably refining function until other selective pressures balance out the tendency to change. Natural selection therefore works as a ratchet, which turns the operation of random variation into a trajectory. In retrospect this may well
look
like a ramp of ascending complexity.